Foods to help balance fatty liver
1. What is fatty liver disease?
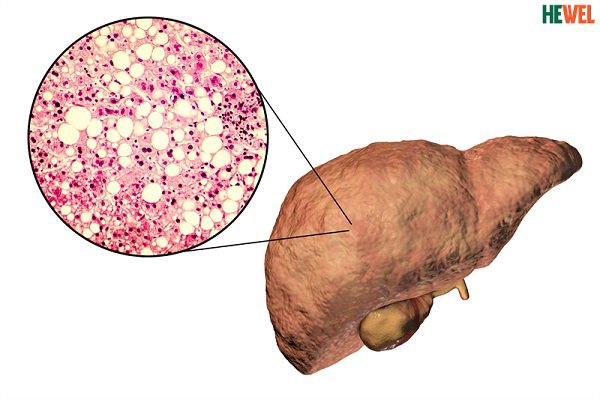
Fatty liver is a common disease with an increasing incidence. Fatty liver disease, if not detected and treated early, can lead to hepatitis, cirrhosis, and even liver cancer. Therefore, it is best to prevent disease with foods that help balance fatty liver.
2. Fatty liver eat what?

3. Lifestyle changes in fatty liver disease
Control and maintain ideal weight: Regular monitoring of weight as well as weight gain is essential in controlling fatty liver disease, helping to control fatty liver disease. Regulates excess fat accumulation in the liver. When you are overweight or obese, you need to actively lose weight to achieve your ideal weight.
The BEST Drink for a Fatty Liver
Dandelion root tea is also believed by some to help detoxify the liver and relieve symptoms of liver disease. To make dandelion tea, you can steep one tablespoon of dandelion roots or flowers in five ounces of boiling water for 30 minutes, then strain or drink the mixture.
Easy Liver Cleanse Recipe: Detox Naturally with Lemon and Olive Oil
You only need to look at simple liver cleanse recipes that combine the flavorful combination of lemon and olive oil. This cleansing method supports liver function and promotes general well-being by utilizing the natural detoxifying qualities of these herbs in a gentle yet effective way.
Olive oil and lemon are the main ingredients in the main cuisine of European restaurants. Many people say that the combination of lemon juice and olive oil can treat many diseases like gallbladder stones.
Furthermore, research has looked at the possible health benefits of the components in lemon juice and olive oil independently.
This article will talk about the fact that studies support the purported health benefits of combining lemon juice with olive oil.
We’ll also review the olive oil lemon juice liver cleanse recipe.
Discover the Mutual Impact of Olive Oil and Lemon on liver
There we will learn about the combination of lemon juice and olive oil. Lemon and olive oil make an extremely unique combination. It is open to all, committed, and productive for the skin, liver, and even blood flow.
Let’s first explore the two fruits independently: The ability of lemon to act as an acidifying agent is its power.
The human body can become acidified if its pH level is out of balance, which can have major negative effects on health. Hyperacidity is countered and the pH value is adjusted during alkalizing. Therefore, sick cells typically lose their strength as well as their breeding ground.
There are many liver cleansing recipes with lemon juice and olive oil. Because these are the most easily used liver detox. Lemon Liver detox works properly to clean liver stones and many other harmful small agents.
Olive oil and Lemon Juice Liver Cleanse Recipe

Ingredients:
- 1/2 cup of extra virgin olive oil
- 1/4 cup of freshly squeezed lemon juice
- 1-2 cloves of minced garlic
Instructions:
- Before beginning the cleanse, fast for a minimum of 12 hours to enable your liver to get ready for detoxification.
- In a bowl, thoroughly mix the olive oil, lemon juice, and minced garlic.
- During one to two hours, take one tiny serving (about one to two tablespoons) of the olive oil mixture every fifteen minutes.
- After you’ve finished the olive oil mixture, lie on your right side with your knees pulled up to your chest for half an hour.
- Spend the rest of the evening sleeping so that the cleanse has time to work.
How do you know that this combination is best for you?
Some research shows that eating lemon juice and olive oil together has health benefits. Many claim that they can aid in weight loss, treat and prevent gallstones, and perform cleanses and detoxes.
Let’s look at each of these claims properly.
Cleansing and Detoxification Ideas
A fast internet search will bring up a variety of recipes claiming to use lemon juice, olive oil, or both to detox and cleanse. It is said that waste and toxins that have accumulated in your body over time are taken out by cleanses and detoxes.
On the other hand, research on the potential cleansing and detoxifying effects of lemon juice and olive oil does not seem to be very extensive.
When compared to those who consumed other plant oils, the researchers discovered that those who consumed olive oil during the study period had greater levels of HDL (good) cholesterol and lower levels of LDL (bad) cholesterol in their blood.
In this article, we will give you the natural liver detox recipe for your best hygiene.
However, the polyphenols and antioxidants in lemon juice and olive oil could be referred to as “cleansing” because they “clean up” or neutralize dangerous free radicals. Which otherwise damages cells and may be a factor in sickness and disease.
Lemon and olive oil are recommended as the best quick liver detox. The human body uses a variety of biological processes to get rid of pollutants and keep itself operating at its best.
I suggest eating a diet that is rich in fruits, vegetables, whole grains, legumes, nuts, seeds, and lean protein sources to support your body’s optimal functioning.
Weight Loss
Vitamin C is rich in lemon juice. 38.7 mg, or 43% of the Recommended Dietary Allowance (RDA) for men and 52% of the RDA for women, is present in a 3-ounce (100-gram) serving. Vitamin C plays a crucial role in the body’s synthesis of carnitine.
A substance called carnitine is responsible for moving fat molecules into cells so they may be broken down and used as an energy source. Thus, a low vitamin C consumption could result in less fat being broken down. For
weight loss, many quick liver detox drinks are also available in the market.
Health Benefits of using Liver oil and Lemon Cleanse
What health advantages are associated with gallbladder cleansing?
To prevent gallstones, certain supporters of alternative healthcare advise liver cleansing. They maintain that the liver releases the gallstones as a result of the liver cleaning.
The gallstones should then ideally pass via the stool. This would mean that the patient would have fewer gallstones left to produce uncomfortable symptoms and might be able to avoid surgery.
There are various kinds of liver cleanses. Alternative medicine practitioners provide many ” liver detox recipes” and traditional treatments online.
- Olive oil and lemon juice. Using this method, you will fast for 12 hours during the day and then drink 4 tablespoons of olive oil and 1 tablespoon of lemon juice, eight times every 15 minutes, starting at 7 p.m.
- Vegetables and apple juice. Using this method, all you consume till five o’clock in the evening is apple and vegetable juice. Every fifteen minutes after 5 p.m., ingest eight ounces of olive oil by consuming 18 milliliters (ml) of olive oil mixed with 9 ml of lemon juice.
In these points, we tell you about the easy liver detox remedy that you can use at home easily.
How might a liver cleanse cause side effects?
Depending on the “recipe” one follows, cleansing may have different adverse effects. For example, a lot of individuals cleanse their livers using olive oil. When consumed in excess, this may have a laxative effect.
The following symptoms are possible for some people to experience after a gallbladder cleanse:
- Diarrhea vomiting nausea
- Depending on the herbs or other things someone utilizes in their cleanse, there could be additional negative consequences.
Furthermore, a person may perform a gallbladder cleansing yet still not be able to get rid of their gallstones. To prevent their symptoms from getting worse or an infection in their gallbladder. They probably need to have surgery at that point.
How to liver cleanse at home?
To support your liver’s natural detoxification activities and enhance general well-being. Liver cleansing at home might be a quick and easy solution. A possible strategy is to consume fewer processed meals, alcoholic beverages, and added sugars while increasing your intake of foods high in fiber, such as fruits, vegetables, and whole grains.
Furthermore, drinking lots of water throughout the day to stay hydrated promotes liver function and the removal of toxins. Herbal teas that you can drink regularly, like milk thistle or dandelion root, are also well-known for their liver-cleansing qualities. Different liver detox tea recipes are also present on the internet.
How much olive oil for detox?
Depending on things like tolerance levels, individual health status, and particular detox procedures, there are differences in the suggested quantity of olive oil for detoxification. In general, it’s important to balance safety and efficacy while using olive oil in a detox program.
As part of a detox treatment, it is generally recommended to ingest 1-2 tablespoons of extra virgin olive oil daily.
The body’s natural detoxification processes are supported by this moderate amount of beneficial substances, which do not overburden the system. These components include antioxidants and monounsaturated fats.
Conclusion
Cleanse Your Liver
In addition to lifestyle changes, nutrition can have an impact on liver health. Check out these beverages that can aid in improving liver function.
Your liver plays many important roles in your physical health. It aids in digestion and metabolism and acts as a filter for the blood, breaking down harmful substances into waste that is expelled from the body through urine and stool.
According to the Mayo Clinic, symptoms of liver dysfunction include:
- Skin and eyes that appear yellowish (jaundice)
- Abdominal pain and swelling
- Swelling in the legs and ankles
- Itchy skin
- Dark urine color
- Pale stool color
- Chronic fatigue
- Nausea or vomiting
- Loss of appetite
- Tendency to bruise easily
Liver disease can be genetic. Other factors like viral infections, age, obesity, or excessive alcohol use may also cause liver damage or dysfunction. If left untreated, this damage can be fatal.
The good news is the liver is the only organ in the body with the ability to regenerate new cells and repair damaged ones. Repairing liver damage can be challenging; however, it is possible through consistent diet and lifestyle changes. These include:
- Maintaining a healthy weight
- Eating nutritious food and having a balanced diet
- Getting regular exercise
- Avoiding harmful additives and chemicals
- Avoiding excessive or continued alcohol or illegal drug use (damage from alcohol abuse can cause irreversible cirrhosis of the liver)
Also, many beverages, such as water, tea, and grapefruit juice, can be beneficial for your overall health and may aid detoxification of the body and liver.
Water
Staying properly hydrated is an important factor in maintaining a healthy liver. Dehydration can greatly affect liver function, especially the ability to detoxify blood. On average, you should drink eight to ten glasses of water a day; those with health conditions may need to increase their water intake beyond the recommended amount.
Teas
There are a few natural teas that may assist in liver function. Several popular and possibly beneficial teas for liver health include:
- Lemon Ginger Tea – reduces the risk of liver disease
- Peppermint Tea – improves digestion and detoxifying functions of the liver
- Green Tea – reduces the accumulation of lipids in the liver and contains antioxidants
Research the health effects and benefits of specific teas or discuss recommendations with your health care provider to ensure safe recommended use.
Grapefruit Juice
Grapefruit juice contains specific antioxidants that stimulate the liver and help filter and excrete chemicals from the body. Grapefruit also contains flavonoids naringin and naringenin, which have anti-inflammatory and antioxidant properties that may help protect the liver. However, it is recommended to not consume more than six ounces of grapefruit juice per day. Grapefruit juice also interacts with many prescription medications, so please check with your doctor or pharmacist and read your medication warning labels before adding it to your diet.
Turmeric Water
Turmeric is a commonly used supplement that may decrease inflammation and assist with liver repair, due to its ability to help flush out harmful toxins while decreasing fat buildup in the liver. For safe use, medical studies recommend mixing one to three grams of dried turmeric root in hot water each day for up to three months.
Lemon Water
Many citrus fruits, including lemon, can be added to water to help stimulate and flush out the liver. Lemons are high in nutrients like vitamin C and antioxidants. To help prevent liver disease, enjoy four to six tablespoons of lemon juice mixed with water each day.
Ginger Water
Ginger helps protect your liver and reduces inflammation in the body. It may also boost immunity and improve digestive health. The recommended consumption is less than four grams of ginger per day, mixed with warm or cold water.
As with any change in nutritional intake or supplement use, please use caution. Some people may experience unwanted results or side effects, and some herbs and foods may interact with certain medications. Also, the information presented above is based on adult studies and should not be used for children. Research any supplements and consult a health care professional before making abrupt changes to your diet or lifestyle. source
DRINK 1 CUP PER DAY to Remove Fat from Your Liver – Dr. Berg
Many people have a fatty liver and don’t even know it.
Today I want to help you better understand the liver and how to remove fat from the liver. The purpose of the liver is to break down toxins into harmless particles. A fatty liver can become heavy and enlarged. This can lead to fullness under the right rib cage and shoulder pain.
Important functions of the liver:
- It makes bile
- It helps convert thyroid hormones
- It buffers excess sex hormones
Bile is made by the liver, and it helps you break down fat. A lack of bile can lead to a lot of various health problems. Bile is also very important to keep fat out of the liver. Anything you can do to help increase bile reserves will help you remove liver fat. You may even want to take purified bile salts. Keep in mind that the ketogenic diet and intermittent fasting can help remove fat from the liver. I believe this is essential if you have a fatty liver.
A great shake to keep fat off the liver: Ingredients (organic if possible):
- 2 cups kale (frozen)
- 1 cup blueberries (frozen)
- 1 cup water
- 1 cup plain whole-milk kefir (organic and grass-fed if possible)
Instructions: Blend all of the ingredients in a blender for a couple of minutes. Drink once a day in combination with a Healthy Keto diet and intermittent fasting.
Protective Effects of Lemon Juice on Alcohol-Induced Liver Injury in Mice
Abstract
Chronic excessive alcohol consumption (more than 40-80 g/day for males and more than 20-40 g/day for females) could induce serious liver injury. In this study, effects of lemon juice on chronic alcohol-induced liver injury in mice were evaluated. The serum biochemical profiles and hepatic lipid peroxidation levels, triacylglycerol (TG) contents, antioxidant enzyme activities, and histopathological changes were examined for evaluating the hepatoprotective effects of lemon juice in mice. In addition, the in vitro antioxidant capacities of lemon juice were determined. The results showed that lemon juice significantly inhibited alcohol-induced increase of alanine transaminase (ALT), aspartate transaminase (AST), hepatic TG, and lipid peroxidation levels in a dose-dependent manner. Histopathological changes induced by alcohol were also remarkably improved by lemon juice treatment. These findings suggest that lemon juice has protective effects on alcohol-induced liver injury in mice. The protective effects might be related to the antioxidant capacity of lemon juice because lemon juice showed in vitro antioxidant capacity.
1. Introduction
Alcohol abuse and alcoholism could lead to serious health and socioeconomic problems worldwide. Chronic excessive alcohol consumption (more than 40–80 g/day for males and more than 20–40 g/day for females) could lead to several illnesses, such as gastrointestinal damage, pancreatitis, alcoholic liver disease, neurologic disorders, diabetes mellitus, and cancer [1, 2]. Among these diseases, alcoholic liver disease has attracted more attention due to its high morbidity and mortality. Alcoholic liver disease is a major type of chronic liver disease throughout the world and can progress to liver cirrhosis and liver cancer.
Chronic alcohol consumption can generate abundant reactive oxygen species (ROS), including superoxide anion radical (O2−•), hydroxyl radical (OH•), and hydrogen peroxide (H2O2). The ROS can react with most cellular macromolecules and subsequently cause cellular damage [3]. Therefore, the excessive ROS induced by alcohol is regarded as an important factor in the development of alcohol-induced liver injury. Various enzymatic and nonenzymatic antioxidants are related to protecting cells against ROS. Antioxidant enzymes include catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx), and nonenzymatic antioxidants include glutathione (GSH), vitamin E, ascorbate, vitamin A, and ubiquinone [4]. Nonenzymatic antioxidants can be enhanced by antioxidant intake. In recent years, many natural products that have abundant antioxidants were reported to possess the effect of scavenging free radicals and protecting the liver from oxidative damage [4, 5].
Lemon is a popular fruit consumed as juice and contains high contents of vitamins and polyphenols (mainly flavonoids), such as hesperidin, eriocitrin, naringin, neohesperidin, rutin quercetin, chlorogenic acid, luteolin, and kaempferol [6]. The in vivo and in vitro experiments have shown that lemon has various health benefits, such as anticancer effect, antimicrobial effect, lipid-lowering effect, and protective effect against cardiovascular diseases [6]. In addition, lemon is used to treat liver ailments in tribal medicine [7]. However, effects of lemon juice on chronic alcohol-induced liver injury have not been reported in the literature. The objective of this study is to investigate the effects of lemon juice on chronic alcohol-induced liver injury in mice. In addition, the in vitro antioxidant capacities of lemon juice were evaluated. The results of this study could supply valuable information for the general public to reduce harm of alcohol consumption.
2. Materials and Methods
2.1. Chemicals and Reagents
The compounds 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,4,6-tri(2-pyridyl)- S-triazine (TPTZ), quercetin, gallic acid, and Folin–Ciocalteu’s phenol reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA). Assay kits for the determination of SOD, lipid peroxidation, CAT, and TG contents were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Other chemicals were of analytical grade.
2.2. Materials
Lemon was obtained from markets in Guangzhou, China. The fruit was cleaned with deionized water. The edible portion was weighed precisely and mixed with deionized water (1 : 1, m/v), and the mixture was ground into a homogenate with a homogenizer. Then, the homogenate was centrifuged at 5,000g for 10 min, and the supernatant was obtained. The supernatant was used for the measurement of antioxidant capacity, total phenolic contents (TPC), and total flavonoid contents (TFC) and for animal experiments. Moreover, in animal experiments, the original supernatant and the diluted supernatant (1 : 5 and 1 : 10, m/v) were used as the high, medium, and low dose, respectively. The lemon juice was freshly prepared before gavage every time.
2.3. Animal Study
Male C57BL/6 mice (20–25 g) were employed in this study. Thirty mice were randomly divided into 5 groups, each group containing 6 mice. They were maintained in a SPF laboratory animal room, which kept a 12 h light/dark cycle at 22 ± 0.5°C with 40%–60% relative humidity. The animal study was performed according to the “Principles of Laboratory Animal Care” and approved by the Institutional Animal Ethics Committee of Sun Yat-sen University. The model group was treated daily with ethanol and distilled water (10 mL/kg) at the same time; the lemon juice treatment groups were treated daily with different concentrations (high dose 1 : 1 (m/v), medium dose 1 : 5, and low dose 1 : 10) of lemon juice (10 mL/kg) and ethanol simultaneously; the control group was treated daily with isometric distilled water. The model group and the lemon juice treatment groups were given ethanol according to the following ways: 35% ethanol (v/v) at a dose of 3 g/kg body weight for 7 days, 40% ethanol (v/v) at a dose of 4 g/kg body weight for the next 7 days, and 52% ethanol (v/v) at a dose of 5 g/kg body weight on the 15th day [8]. All the intervention methods were intragastric administration. The blood and liver were collected from mice 9 h after the last ethanol administration. The blood sample was centrifuged at 4,000g for 10 min and the serum was collected. The obtained serums were stored at −22°C before determination. A piece of tissue was taken from liver and fixed in 4% paraformaldehyde, and then the remaining liver tissue was stored at −22°C until use.
2.4. Measurement of Biochemical Parameters in the Serum
The levels of ALT, AST, and TG in serum were determined by a Hitachi-7180 automated biochemistry analyzer (Hitachi, Japan) with the corresponding reagent kit.
2.5. Measurement of TG and Antioxidant Enzyme Activities in the Liver
The levels of TG, SOD, and CAT in liver tissue were measured using the commercial detection kits according to the manufacturer’s instructions.
2.6. Measurement of Lipid Peroxidation Levels in the Liver
The levels of lipid peroxidation in liver tissue were measured by thiobarbituric acid (TBA) method using the commercial detection kits according to the manufacturer’s instructions. The reference standard was malondialdehyde (MDA), and the results were expressed as nmol MDA equivalent/mg prot.
2.7. Liver Histopathological Assessment
The liver tissue fixed in 4% paraformaldehyde was embedded in paraffin, sectioned into 5 μm thickness, and stained with hematoxylin-eosin (H&E) for evaluation of histopathological changes. The histopathological changes of stained liver slices were observed under a bright-field microscope.
2.8. Ferric-Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was performed based on the method described in the literature [9]. In brief, the FRAP reagent was prepared from 10 mmol/L TPTZ solution, 20 mmol/L iron(III) chloride solution, and 300 mmol/L sodium acetate buffer solution (pH 3.6) in a volume ratio of 1 : 1 : 10, respectively. 100 μL of the diluted sample was added to 3 mL of the FRAP reagent and the mixture was measured after 4 min at 593 nm. The standard curve was established using FeSO4 solution, and the results were expressed as μmol Fe(II)/g dry weight of lemon.
2.9. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
The TEAC assay was carried out according to the procedure in the literature [10]. Briefly, the ABTS•+ stock solution was prepared from 2.45 mmol/L potassium persulfate and 7 mmol/L ABTS solution in a volume ratio of 1 : 1 and then placed in the dark for 16 h at room temperature. The ABTS•+ working solution was prepared by diluting the stock solution, and the absorbance of ABTS•+ working solution was 0.710 ± 0.05 at 734 nm. 100 μL of the diluted sample was mixed with 3.8 mL ABTS•+ working solution, and the absorbance of the mixture was measured at 734 nm after 6 min, and the percent of inhibition of absorbance at 734 nm was calculated. The reference standard was Trolox, and the results were expressed as μmol Trolox/g dry weight of lemon.
2.10. Determination of TPC
TPC were measured according to the literature [11]. Briefly, 0.50 mL of the diluted sample was added to 2.5 mL of 0.2 mmol/L Folin–Ciocalteu reagent. After 4 min, 2 mL of saturated sodium carbonate solution was added. After incubation for 2 h at room temperature, the absorbance of the mixture was measured at 760 nm. The reference standard was gallic acid, and the results were expressed as mg gallic acid equivalent (GAE)/g dry weight of lemon.
2.11. Determination of TFC
TFC were measured according to the literature [12]. In brief, 0.50 mL of the sample was mixed with 1.5 mL of 95% ethanol (v/v), 0.1 mL of 10% aluminum chloride (w/v), 0.1 mL of 1 mol/L potassium acetate, and 2.8 mL of water. After incubation for 30 min at room temperature, the absorbance of the mixture was determined at 415 nm. The reference standard was quercetin, and the results were expressed as mg of quercetin equivalent (QE)/g dry weight of lemon.
2.12. Statistical Analysis
Statistical analysis was carried out by one-way analysis of variance (ANOVA) with post hoc LSD test using SPSS 13.0 software. p < 0.05 was regarded as significant.
3. Results
3.1. Effects of Lemon Juice on the Levels of ALT and AST in Serum
As shown in Figure 1, the administration of alcohol led to a significant (p < 0.05) elevation of alanine transaminase (ALT) and aspartate transaminase (AST) levels in serum of the model group compared with that of the control group. The administration of low and medium concentration of lemon juice slightly prevented the elevation of serum level of AST, while a high dose of lemon juice significantly (p < 0.05) decreased it. At the same time, the prevention of the elevation of serum levels of ALT was observed significantly (p < 0.05) in medium and high concentration of lemon juice group and displayed a dose-effect relationship.
Effects of lemon juice on the levels of AST (a) and ALT (b) in serum of mice. Control: normal group; Model: alcohol group; LL: alcohol and low dose of lemon juice group; LM: alcohol and medium dose of lemon juice group; LH: alcohol and high dose of lemon juice group. ∗ means the levels of parameters in the model group were significantly (p < 0.05) different from those of the control group. # means the levels of parameters in the treatment group were significantly (p < 0.05) different from those of the model group.
3.2. Effects of Lemon Juice on the Levels of TG in Serum and Liver
Triacylglycerol (TG) content in serum was significantly (p < 0.05) increased in the model group compared with that in the control group (Figure 2(a)). Administration of lemon juice reduced the accumulation of TG in a dose-dependent manner, especially in high concentration of lemon juice group (p < 0.05). In addition, hepatic TG content was significantly (p < 0.05) increased in model group compared with that in the control group (Figure 2(b)). Administration of medium and high concentration of lemon juice significantly (p < 0.05) reduced the accumulation of hepatic TG in a dose-dependent manner.
Effects of lemon juice on TG contents in serum (a) and liver (b). Control: normal group; Model: alcohol group; LL: alcohol and low dose of lemon juice group; LM: alcohol and medium dose of lemon juice group; LH: alcohol and high dose of lemon juice group. ∗ means the levels of parameters in the model group were significantly (p < 0.05) different from those of the control group. # means the levels of the parameters in the treatment group were significantly (p < 0.05) different from those of the model group.
3.3. Effects of Lemon Juice on Liver Lipid Peroxidation Levels
The lipid peroxidation levels in liver tissue are shown in Figure 3. Compared with that of the control group, there was a significant (p < 0.05) increase in the lipid peroxidation level of the model group. The administration of lemon juice significantly (p < 0.05) decreased the level of lipid peroxidation in a dose-dependent manner.

Effects of lemon juice on hepatic lipid peroxidation level in mice. Control: normal group; Model: alcohol group; LL: alcohol and low dose of lemon juice group; LM: alcohol and medium dose of lemon juice group; LH: alcohol and high dose of lemon juice group. ∗ means the levels of the parameters in the model group were significantly (p < 0.05) different from those of the control group. # means the levels of the parameters in the treatment group were significantly (p < 0.05) different from those of the model group.
3.4. Effects of Lemon Juice on Liver Antioxidant Enzyme Activities
Figure 4 represents the results of hepatic antioxidant enzyme activities in five groups. The SOD level in the liver increased significantly (p < 0.05) in the model group compared with that in the control group. The CAT level in the liver decreased only slightly (p > 0.05) in the model group compared with the control group in this study. However, treatment with lemon juice significantly (p < 0.05) decreased the levels of SOD and CAT compared with those of the model group. In addition, all the biochemical parameters are summarized in Table 1.
Effects of lemon juice on the activities of SOD (a) and CAT (b) in liver. Control: normal group; Model: alcohol group; LL: alcohol and low dose of lemon juice group; LM: alcohol and medium dose of lemon juice group; LH: alcohol and high dose of lemon juice group. ∗ means the levels of the parameters in the model group were significantly (p < 0.05) different from those of the control group. # means the levels of the parameters in the treatment group were significantly (p < 0.05) different from those of the model group.
Table 1
Effects of lemon juice on the levels of several biochemical parameters.
| Parameters | Control | Model | LL | LM | LH |
|---|---|---|---|---|---|
| AST (U/L) | 103 ± 10.45 | 136.53 ± 19.94∗ | 117.88 ± 15.37 | 113.5 ± 7.7 | 98.85 ± 10.94# |
| ALT (U/L) | 40.5 ± 3.89 | 54.32 ± 4.76∗ | 54.05 ± 7.18 | 41.32 ± 6.25# | 34.68 ± 2.71# |
| Serum TG (nmol/L) | 0.4 ± 0.06 | 1.01 ± 0.12∗ | 1.09 ± 0.04 | 1.03 ± 0.05 | 0.82 ± 0.08# |
| Liver TG (mmol/g prot) | 0.07 ± 0.01 | 0.1 ± 0.02∗ | 0.09 ± 0.01 | 0.07 ± 0.01# | 0.06 ± 0.01# |
| Lipid peroxidation (nmol MDA equivalent/mg prot) | 0.64 ± 0.14 | 1.26 ± 0.22∗ | 0.88 ± 0.12# | 0.84 ± 0.15# | 0.72 ± 0.13# |
| SOD (U/mg prot) | 89.6 ± 3.42 | 97.51 ± 3.96∗ | 85.27 ± 5.57# | 83 ± 9.28# | 81.03 ± 6.65# |
| CAT (U/mg prot) | 6.55 ± 0.41 | 6.29 ± 0.39 | 5.55 ± 0.64# | 5.47 ± 0.28# | 5.17 ± 0.51# |
Note. Control: normal group; Model: alcohol group; LL: alcohol and low dose of lemon juice group; LM: alcohol and medium dose of lemon juice group; LH: alcohol and high dose of lemon juice group. ∗ means the levels of the parameters in the model group were significantly (p < 0.05) different from that of the control group. # means the levels of the parameters in the treatment group were significantly (p < 0.05) different from that of the model group.
3.5. Histopathological Evaluation
Histopathology assessment of the liver was carried out for all groups (Figure 5). There was no pathological abnormality observed in the liver of the control group with preserved cytoplasm and distinct nucleus as shown in Figure 5(a). In Figure 5(b), it was observed in the model group that ethanol induced necrosis changes and substantial small fat droplets changes in liver section. However, livers of mice in all lemon juice treated groups showed noticeable recovery from ethanol induced liver damage with fewer small fat droplets changes and hepatocytes necrosis features.
The photomicrographs of liver sections taken from mice. (a) Normal group; (b) alcohol group; (c) alcohol and low dose of lemon juice group; (d) alcohol and medium dose of lemon juice group; (e) alcohol and high dose of lemon juice group. Arrow indicates a condition of small fat droplets changes, and the circle indicates hepatocytes necrosis, which mainly occurs in alcohol model group.
3.6. The In Vitro Antioxidant Activity, Total Phenolic Contents (TPC), and Total Flavonoid Contents (TFC) of Lemon Juice
The in vitro antioxidant activities of lemon were evaluated using ferric-reducing antioxidant power (FRAP) and Trolox equivalent antioxidant capacity (TEAC) assays. The FRAP and TEAC values were 50.82 ± 2.70 μmol Fe(II)/g dry weight (DW) and 19.88 ± 0.66 μmol Trolox/g DW, respectively. The total phenolic contents (TPC) and total flavonoid contents (TFC) of lemon were 6.21 ± 0.28 mg GAE/g DW and 0.30 ± 0.03 mg QE/g DW, respectively.
4. Discussion
Alcohol use disorder causes substantial diseases, and the liver is the most adversely affected organ. In the present study, the effects of lemon juice on chronic alcohol-induced liver injury in mice were investigated. Ethanol induced impairment of liver in mice was evidenced by increased AST and ALT levels. Treatment with lemon juice lowered the increased levels of AST and ALT in serum. The return of the activities of aminotransferases (AST or ALT) in serum to normal indicates the regeneration of hepatocytes and the healing of hepatic parenchyma; therefore, lemon juice had a protective effect on alcohol-induced liver injury. The results were in agreement with previous reports that showed lemon possessing a hepatoprotective effect on liver injury induced by carbon tetrachloride and acute exercise [7, 13]. In addition, the chronic alcohol-induced liver damage was further confirmed by liver histopathological changes in the present study, and treatment with lemon juice also remarkably improved the liver histopathological changes, which further confirmed the hepatoprotective activity of lemon juice on alcohol-induced liver injury in mice.
Various factors and mechanisms are associated with the pathological progress of alcohol-induced liver injury, and oxidative stress was one of them [3]. ROS is one kind of prooxidants including hydroxyl radical, superoxide radical, and hydrogen peroxide, which are frequently generated spontaneously during metabolism. Normally produced ROS is rapidly eliminated by the antioxidant defense system. The antioxidant defense system is able to scavenge ROS and terminate chain reaction of free radicals in vivo. Alcoholic exposure can result in excessive accumulation of ROS and contribute to cellular damage. Excessive accumulation of ROS could cause lipid peroxidation of hepatocytes, which was regarded as the primary mechanism concerned with chronic alcohol-induced liver damage [8]. MDA, the product of lipid peroxidation induced by ROS, also accumulates in the alcohol-damaged liver and represents a good estimation of the total oxidative stress [3]. In the present study, alcohol significantly augmented lipid peroxidation levels, which was similar to the previous study that showed increased lipid peroxidation in alcoholic patients [14]. Treatment with lemon juice reduced the level of lipid peroxidation to a normal level, which showed a significant protective effect of lemon juice against alcohol-induced oxidative stress.
Liver steatosis is the earliest disease of the liver on account of chronic ethanol consumption, with the characteristic of fat accumulation. It is generally accepted that, in the development of hepatic steatosis, ethanol exposure increases the ratio of reduced nicotinamide adenine dinucleotide/oxidized nicotinamide adenine dinucleotide in hepatocytes, which disturb mitochondrial fatty acid β-oxidation and induce steatosis further [15]. In this study, alcohol-induced occurrence of hepatic steatosis was confirmed by increased hepatic TG contents and histopathological changes. Treatment with lemon juice significantly lowered the hepatic TG contents and improved the damaged histopathological changes. In particular, the mice given high dose of lemon juice had almost completely recovered to normal.
The antioxidant enzymes, such as SOD and CAT, represent the defense response system to excessive ROS. SOD catalyzes the dismutation of two superoxide anions to hydrogen peroxide and oxygen, and then CAT degrades two hydrogen peroxide molecules to water and oxygen [16]. SOD is also considered as front line among antioxidant enzymes in defense against free radicals. In the literature, the effects of alcohol treatment on the levels of SOD/CAT are controversial. SOD showed an increase, no changes, or a decrease, depending on the model, diet, duration, and amount of alcohol consumption [17–19]. In addition, it was reported that CAT activity decreased upon chronic ethanol consumption in a study [20]. However, another study showed that CAT activity was increased in rat liver [18]. In our study, the alcohol treatment significantly increased the activity of SOD and slightly decreased the activity of CAT, while treatment with lemon juice decreased the activities of SOD and CAT. The increased activity of SOD reflects the activation of the compensatory mechanism which might be an attempt to counteract free radicals in the liver [21]. The treatment with lemon juice prevented ROS accumulation, and the compensatory effects were not available in the liver. Therefore, lemon juice decreased the activities of SOD and CAT. The results were similar to the report of Gasparotto et al. [22]. In addition, the in vitro antioxidant experiment of lemon also showed that lemon had medium in vitro antioxidant capacities, which contribute to the explanation of the in vivo free radical scavenging effect of lemon.
Lemon contains numerous beneficial bioactive compositions, including phenolic compounds (mainly flavonoids), vitamins, carotenoids, essential oils, minerals, and dietary fiber [6]. The hepatoprotective effect of lemon may be attributable to the presence of vitamins, flavonoids, essential oils, and pectin. Vitamin C, a water-soluble antioxidant in lemon, is in a unique position to scavenge aqueous peroxyl radicals and react with free radicals, thus preventing oxidative damage including lipid peroxidation [14]. Sometimes, vitamin C could exert prooxidative effects at low concentrations and in the existence of transition metal ions [23], which might aggravate oxidative stress. However, it is difficult for vitamin C to have prooxidative effects in vivo due to the presence of NADPH-dependent recycling systems and glutathione [24]. In addition, there were some literatures reporting that vitamin C supplementation alone could reduce oxidative stress induced by ethanol, and the hepatoprotective effect of vitamin C treatment was more effective than silymarin, quercetin, and thiamine [25, 26]. Flavonoids, a class of secondary plant phenolics, can interact with hydroxyl radicals, chelate metal catalysts, and inhibit oxidases [27]. In previous studies, lemon flavonoid was shown to possess a hepatoprotective effect on liver damage induced by carbon tetrachloride and acute exercise, and the mechanism of the protective effect was related to the antioxidant capacity [7, 13]. Lemon essential oils and pectin were found to have protective effects on stomach and intestine barrier function [28, 29]. Ethanol exposure can injure the defensive intestinal barrier and increase the permeability of the small intestine, which lead to bacterial endotoxins leakage [25]. The bacterial endotoxins leakage is an important factor in the pathogenesis of alcohol-induced liver injury [30]. Therefore, the lemon essential oils and pectin might protect the intestine barrier function, thus indirectly protecting against alcohol-induced liver injury.
In this study, lemon juice revealed a protective effect on chronic alcohol-induced liver injury. Due to the fact that lemon contains a variety of bioactive ingredients, the hepatoprotective effect might be the result of joint action of multiple mechanisms, and it is difficult to clarify the specific mechanism of effect. The medium in vitro antioxidant capacities of lemon and reduced in vivo MDA levels indicated that lemon might reduce the oxidative stress induced by ethanol, thus exerting hepatoprotective effects. This study has found that lemon juice has a strong hepatoprotective effect, which provides valuable information for the general public to reduce harm of alcohol consumption. In the future, active components in lemon juice should be separated and identified, and the mechanism of action of the purified compound should be explored, including the action on the small intestine.
5. Conclusions
Chronic alcohol consumption could induce liver injury. Lemon juice is readily available as a widely consumed beverage. In this study, we found that treatment with lemon juice exerted hepatoprotective effects on alcohol-induced liver injury in mice through decreasing the levels of serum ALT and AST as well as hepatic TG and lipid peroxidation. In addition, the in vitro antioxidant experiment of lemon showed that lemon had medium in vitro antioxidant capacities. Therefore, we speculate that the hepatoprotective effects might be related to the antioxidant capacities of lemon juice. The results showed that lemon juice might be a potential dietary supplement for the prevention and treatment of liver injury related to chronic alcohol consumption.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81372976), Key Project of Guangdong Provincial Science and Technology Program (no. 2014B020205002), and the Hundred-Talents Scheme of Sun Yat-sen University.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
Similar articles
-
The Treatment of Rheumatic Diseases by Lemon-Juice; with Illustrative Cases from Hospital Practice.
Br Foreign Med Chir Rev. 1849 Oct;4(8):529-530.PMID: 30164951 Free PMC article. Review. No abstract available. -
J Agric Food Chem. 2016 Sep 28;64(38):7291-7. doi: 10.1021/acs.jafc.6b02907. Epub 2016 Sep 19.PMID: 27609057
-
Int J Mol Sci. 2015 Jan 22;16(2):2446-57. doi: 10.3390/ijms16022446.PMID: 25622257 Free PMC article.
-
Food Funct. 2012 Jun;3(6):628-34. doi: 10.1039/c2fo10266h.PMID: 22648047
-
Protective effect of bicyclol on acute alcohol-induced liver injury in mice.
Eur J Pharmacol. 2008 May 31;586(1-3):322-31. doi: 10.1016/j.ejphar.2008.02.059. Epub 2008 Feb 29.PMID: 18371952
Cited by
-
Antioxidants (Basel). 2024 May 10;13(5):588. doi: 10.3390/antiox13050588.PMID: 38790693 Free PMC article.
-
Forensic Toxicol. 2024 Jan;42(1):45-59. doi: 10.1007/s11419-023-00674-w. Epub 2023 Oct 9.PMID: 37814103
-
Prevention of the Pro-Aggressive Effects of Ethanol-Intoxicated Mice by Schisandrin B.
Nutrients. 2023 Apr 15;15(8):1909. doi: 10.3390/nu15081909.PMID: 37111128 Free PMC article. -
Cells. 2022 Apr 12;11(8):1305. doi: 10.3390/cells11081305.PMID: 35455983 Free PMC article.
-
Nutrients. 2021 May 11;13(5):1612. doi: 10.3390/nu13051612.PMID: 34064981 Free PMC article. Review.
References
-
- Arteel G., Marsano L., Mendez C., Bentley F., McClain C. J. Advances in alcoholic liver disease. Best Practice and Research in Clinical Gastroenterology. 2003;17(4):625–647. – PubMed
Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver
Abstract
The purpose of this study is to determine the protective effect of Taraxacum official (dandelion) leaf extract (DLE) on high-fat-diet (HFD)-induced hepatic steatosis, and elucidate the molecular mechanisms behind its effects. To determine the hepatoprotective effect of DLE, we fed C57BL/6 mice with normal chow diet (NCD), high-fat diet (HFD), HFD supplemented with 2g/kg DLE DLE (DL), and HFD supplemented with 5 g/kg DLE (DH). We found that the HFD supplemented by DLE dramatically reduced hepatic lipid accumulation compared to HFD alone. Body and liver weights of the DL and DH groups were significantly lesser than those of the HFD group, and DLE supplementation dramatically suppressed triglyceride (TG), total cholesterol (TC), insulin, fasting glucose level in serum, and Homeostatic Model Assessment Insulin Resistance (HOMA-IR) induced by HFD. In addition, DLE treatment significantly increased activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) in liver and muscle protein. DLE significantly suppressed lipid accumulation in the liver, reduced insulin resistance, and lipid in HFD-fed C57BL/6 mice via the AMPK pathway. These results indicate that the DLE may represent a promising approach for the prevention and treatment of obesity-related nonalcoholic fatty liver disease.
Keywords: AMPK; Fatty liver; High-fat diet; Insulin resistance; Taraxacum official (dandelion).
Copyright © 2013 Elsevier Ltd. All rights reserved.
Similar articles
-
Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects.
Molecules. 2023 Jun 27;28(13):5022. doi: 10.3390/molecules28135022.PMID: 37446683 Free PMC article. Review. -
Nutrients. 2022 Mar 24;14(7):1350. doi: 10.3390/nu14071350.PMID: 35405963 Free PMC article. Review.
-
Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity.
Int J Mol Med. 2019 Jan;43(1):603-613. doi: 10.3892/ijmm.2018.3941. Epub 2018 Oct 18.PMID: 30365061 -
Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase.
Basic Clin Pharmacol Toxicol. 2013 Sep;113(3):152-7. doi: 10.1111/bcpt.12076. Epub 2013 May 25.PMID: 23574662 -
Cassia tora (Leguminosae) seed extract alleviates high-fat diet-induced nonalcoholic fatty liver.
Food Chem Toxicol. 2013 Jan;51:194-201. doi: 10.1016/j.fct.2012.09.024. Epub 2012 Sep 28.PMID: 23026700
Cited by
-
Foods. 2023 Oct 17;12(20):3800. doi: 10.3390/foods12203800.PMID: 37893693 Free PMC article.
-
Nutrients. 2023 Sep 24;15(19):4120. doi: 10.3390/nu15194120.PMID: 37836404 Free PMC article.
-
Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects.
Molecules. 2023 Jun 27;28(13):5022. doi: 10.3390/molecules28135022.PMID: 37446683 Free PMC article. Review. -
Nutrients. 2022 Dec 24;15(1):80. doi: 10.3390/nu15010080.PMID: 36615741 Free PMC article.
-
Front Pharmacol. 2022 Sep 6;13:942996. doi: 10.3389/fphar.2022.942996. eCollection 2022.PMID: 36147318 Free PMC article.
- source








