Covid19 Vaccine & Stroke: Cerebral Vein Thrombosis (Stroke) With Vaccine –
Induced Immune Thrombotic Thrombocytopenia
Abstract
In the spring of 2021, reports of rare and unusual venous thrombosis in association with the ChAdOx1 and Ad26.COV2.S adenovirus-based coronavirus vaccines led to a brief suspension of their use by several countries. Thromboses in the cerebral and splanchnic veins among patients vaccinated in the preceding 4 weeks were described in 17 patients out of 7.98 million recipients of the Ad26.COV2.S vaccine (with 3 fatalities related to cerebral vein thrombosis) and 169 cases of cerebral vein thrombosis among 35 million ChAdOx1 recipients. Events were associated with thrombocytopenia and anti-PF4 (antibodies directed against platelet factor 4), leading to the designation vaccine-induced immune thrombotic thrombocytopenia. Unlike the related heparin-induced thrombotic thrombocytopenia, with an estimated incidence of <1:1000 patients treated with heparin, and a mortality rate of 25%, vaccine-induced immune thrombotic thrombocytopenia has been reported in 1:150 000 ChAdOx1 recipients and 1:470 000 Ad26.COV.2 recipients, with a reported mortality rate of 20% to 30%. Early recognition of this complication should prompt testing for anti-PF4 antibodies and acute treatment targeting the autoimmune and prothrombotic processes. Intravenous immunoglobulin (1 g/kg for 2 days), consideration of plasma exchange, and nonheparin anticoagulation (argatroban, fondaparinux) are recommended. In cases of cerebral vein thrombosis, one should monitor for and treat the known complications of venous congestion as they would in patients without vaccine-induced immune thrombotic thrombocytopenia. Now that the Ad26.COV2.S has been reapproved for use in several countries, it remains a critical component of our pharmacological armamentarium in stopping the spread of the human coronavirus and should be strongly recommended to patients. At this time, the patient and community-level benefits of these two adenoviral vaccines vastly outweigh the rare but serious risks of vaccination. Due to the relatively low risk of severe coronavirus disease 2019 (COVID-19) in young women (<50 years), it is reasonable to recommend an alternative vaccine if one is available. Ongoing postmarketing observational studies are important for tracking new vaccine-induced immune thrombotic thrombocytopenia cases and other rare side effects of these emergent interventions.
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected 1 in 50 world citizens and claimed the lives of over 3 million people since December 2019.1 Patients with severe coronavirus disease 2019 (COVID-19) are at high risk of thrombotic complications, with observational studies indicating up to one-third hospitalized with COVID-19 develop acute myocardial injury,2 15% to 30% develop venous thromboembolism (VTE),3 and 1% to 5% experience acute stroke.4–7 It is because of these systemic complications that the mortality rate is 3× that of other viral infections such as seasonal influenza.8
The emergence of SARS-CoV-2 variants has been even more concerning.9 The B.1.1.7 variant, which originated in the United Kingdom, is reported to be more contagious than the original virus described in Wuhan,10 whereas both the B.1.1.7 and B.1.351 variants may be responsible for the lower efficacy of several current vaccines.11–13 A global resurgence in case numbers since February 2021 may be explained by these emerging variants and leniency in public health measures.
At present, 5 vaccines are authorized for emergency use due to proven efficacy in clinical trials14–19 (Table 1). Although randomized trials including >60 000 blinded vaccine recipients have demonstrated a low risk of serious adverse events, reports have emerged of rare thrombotic complications with 2 vaccines. In this commentary, we summarize the known risks of these vaccines compared with the known thrombotic and systemic risks of COVID-19.
| Pfizer14 | Moderna19 | AstraZeneca15 | Johnson & Johnson16 | Gamaleya17 | |
|---|---|---|---|---|---|
| Vaccine name | BNT162b2 | mRNA-1273 | ChAdOx1 | Ad26.COV2.S | Ad26/Ad5 |
| Vector | mRNA | mRNA | Adenovirus | Adenovirus | Adenovirus |
| Dosage | 2 doses, 3 wks apart | 2 doses, 4 wks apart | 2 doses, up to 12 wks apart | 1 dose | 2 doses, 3 wks apart |
| Efficacy against infection | 95% | 95% | 62%–90% | 70% | 92% |
| Efficacy against severe COVID-19 | 95% | 94.1% | Up to 100% | 85% | 90% |
| Storage | −70 °C | −20 °C | 4 °C | 4 °C | 4 °C |
Thrombosis in COVID-19
The thrombotic complications of COVID-19 have been well described.3,4,20,21 As in patients without COVID-19, therapeutic anticoagulation is recommended in patients who develop VTE with COVID-19; however, there is no strong evidence to support the empirical use of therapeutic anticoagulation in unaffected persons.22 The ACTIV-4 trial (Anti-Thrombotics for Adults Hospitalized With COVID-19) comparing therapeutic against prophylactic anticoagulation based on d-dimer level halted enrollment of critically ill patients due to a signal for harm in patients randomized to therapeutic anticoagulation.23 Preliminary data from the REMAP-CAP trial (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia) suggested futility of therapeutic anticoagulation in critically ill patients with COVID-19.24
Among locations for arterial and venous thrombosis in COVID-19, several reports25–27 and a systematic review28 have indicated a small but significant risk of cerebral vein thrombosis (CVT). It is estimated that COVID-19 increases the odds of CVT by >40-fold, affecting as many as 1 in 5000 to 15 000 hospitalized patients with COVID-19,4,29 although the true prevalence among patients with COVID-19 is unknown. More than two-thirds of patients with COVID-19 with CVT lack traditional risk factors,28 with a mortality rate greater than patients without COVID-19 with CVT.28,29
CVT Associated With Adenoviral COVID-19 Vaccines
Early Reports and Event Rates
Since the approval of COVID-19 vaccines in December 2020 by the Food and Drug Administration and European Medicines Agency (EMA), >116 million vaccine sdoses have been administered in Europe and over 215 million doses in the United States, correlating with a significant decline in new case rates and fatalities from COVID-19 (Figure 1).
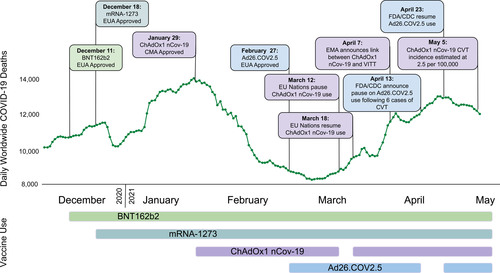
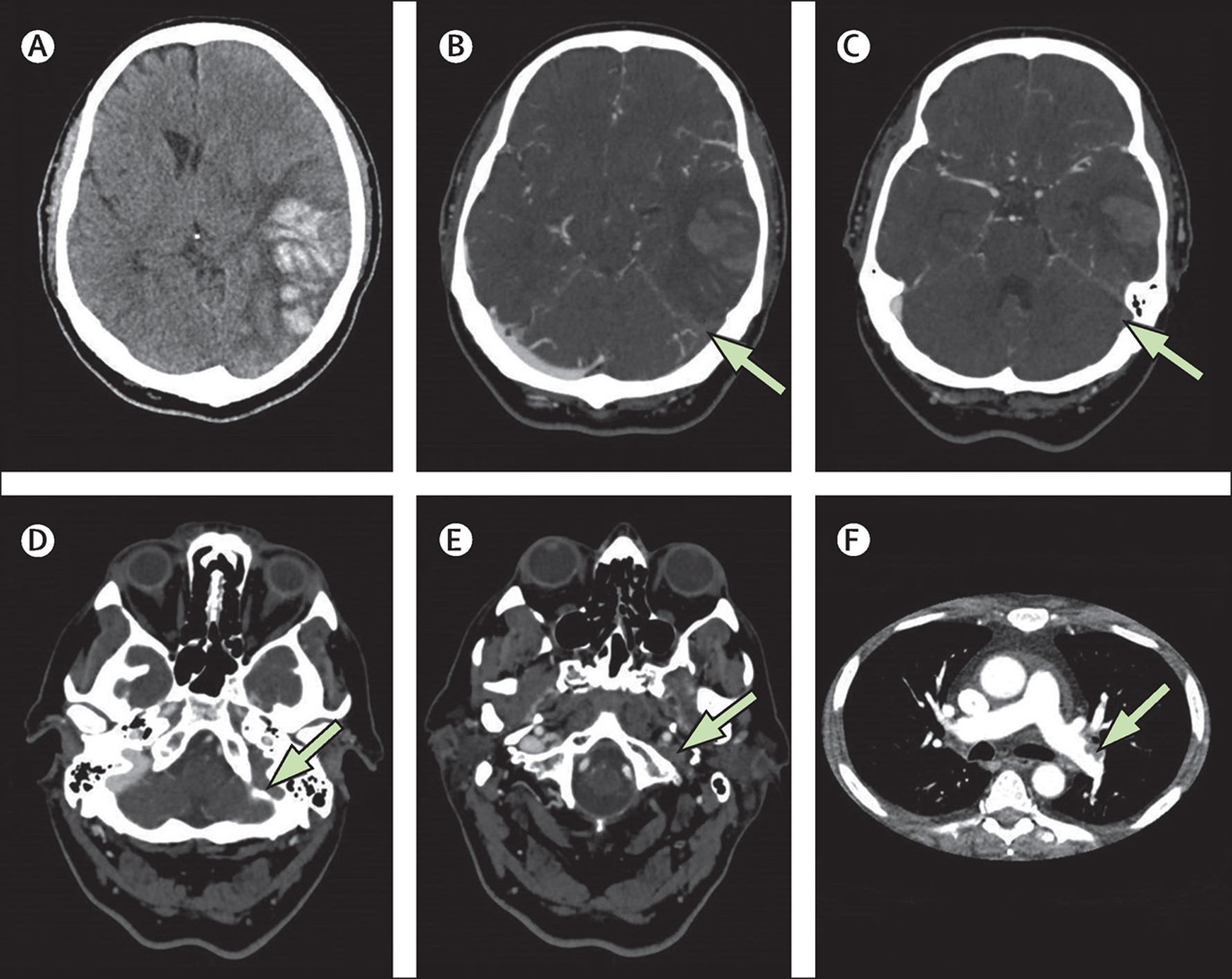
In February 2021, reports emerged of patients with thrombocytopenia and VTE in unusual locations following the ChAdOx1 vaccine, resulting in its suspension in several countries by mid-March. By April 4, 2021, 169 cases of CVT and 53 cases of splanchnic vein thrombosis were reported to the EMA among 35 ChAdOx1 million vaccine recipients.30 On May 5, 2021, a population cohort study in Denmark and Norway reported increased rates of venous thromboembolic events, including CVT, among recipients of the ChAdOx1 vaccine with no increase in arterial events.31 These recent data suggest an excess event rate of CVT of 2.5 per 100 000 ChAdOx1 recipients, although laboratory testing has not confirmed that these events are due to vaccine-induced immune thrombotic thrombocytopenia (VITT).
In the United States, 17 recipients of Ad26.COV2.S (of 7.98 million recipients) were found to develop CVT and thrombocytopenia, with 14 cases of CVT (3 of which were fatal),32 leading the Centers for Disease Control (CDC) and EMA to recommend temporary discontinuation of this vaccine. Twelve of these patients have been described in detail by See et al.33 We have summarized the patient-level data for reported individuals in Table I in the Data Supplement.33–41 Importantly, the majority of decedents were middle-aged or older (median age 69 years, range 21–97), also indicating a low risk of dying from the vaccine among younger recipients.32
The unique presentation of these thrombotic events with thrombocytopenia and the temporal relationship with vaccination has been termed VITT. As more cases have been reported to the EMA and CDC, the estimated incidence of VITT is ≈1 in 150 000 for the ChAdOx1 nCov-19 vaccine and 1 in 470 000 for the Ad26.COV2.S vaccine (Figure 2), although the risks may vary per age group and sex. Although these events may be increasingly recognized, the absolute numbers are small and the epidemiological data continue to overwhelmingly support the safety and efficacy with respect to COVID-19 risk reduction and death due to COVID-19 (or vaccines) among vaccine recipients irrespective of age (Table 2).15,19,32,42
| Unvaccinated mortality rate due to COVID-19 | Mortality rate due to COVID-19 (or vaccine complications) following vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Global COVID-19 mortality rate, % | ChAdOx1 mortality rate15 | Estimated RRR (ChAdOx1) | Ad.26.COV2.S mortality rate32 | Estimated RRR (Ad.26. COV2.S) | BNT162b2 mortality rate42 | Estimated RRR (BNT162b2) | mRNA-1273 mortality rate19 | Estimated RRR (mRNA-1273) | |
| All ages | 2.1% | 0% (95% CI, 0%–0.08% of 5807 recipients) | >95% | 0.001% (95% CI, 8.9×10-6%–0.001% of 8 million recipients) | >99.9% | 0.002% (95% CI, 0.00018%–0.0025% of 6.5 million recipients) | >99.8% | 0% (95% CI, 0%–0.03% of 15 185 recipients) | >98% |
| <29 y | <0.2% | Unknown | Inestimable | Unknown | Inestimable | Age <44: 0% (95% CI, 0%–0.0002% of 2 290 820 recipients) | >99.9% | Age <65: 0% (95% CI, 0%–0.04% of 11 415 recipients) | >98% |
| 30–39 y | <0.2% | Unknown | Inestimable | Unknown | Inestimable | ||||
| 40–49 y | <0.3% | Unknown | Inestimable | Unknown | Inestimable | Age 45–64: 7×10-6% (95% CI, 5×10-6%−0.0001% of 1.8 million recipients) | >99.9% | ||
| 50–59 y | 0.80% | Unknown | Inestimable | Unknown | Inestimable | ||||
| 60–69 y | 2.70% | Unknown | Inestimable | Unknown | Inestimable | Age >64: 0.01% (95% CI, 0.0009%–0.01% of 1.1 million recipients) | >99.8% | Age >64: 0% (95% CI, 0%–0.1% of 3770 recipients) | >95% |
| 70–79 y | 6.50% | Unknown | Inestimable | Unknown | Inestimable | ||||
| >79 y | 14.80% | Unknown | Inestimable | Unknown | Inestimable | ||||
Pathophysiology
Cases of VITT have not been reported following other adenovirus-based vaccines or mRNA-based COVID-19 vaccines. Although adenoviruses are known to activate platelets, it is unlikely that the thrombocytopenia following vaccination is due to the adenoviral vector.15 Additionally, there have been no reported cases of VITT following administration of either the other adenoviral coronavirus vaccines (AD5-nCOV or Gam-COVID-Vac) or adenovirus-based Ebola virus vaccines (Ad5-EBOV and Ad26.ZEBOV).43
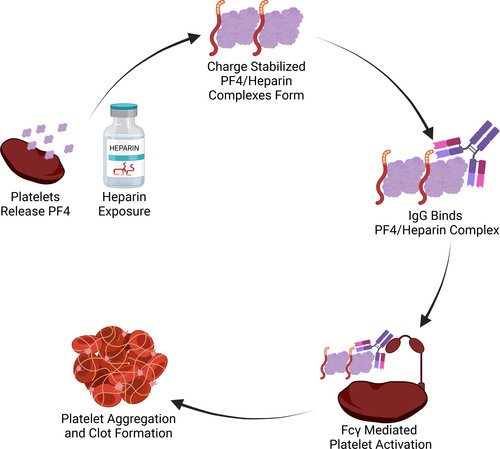
The association of unusual thrombotic complications with thrombocytopenia raised suspicion for inflammatory coagulopathy. Investigators identified antibodies targeting PF4 (platelet factor 4) in the sera of patients with VITT,35 suggesting an autoimmune vaccine response. A similar mechanism has been described in heparin-induced thrombotic thrombocytopenia (HITT; Figure 2).44 In HITT, antibodies are directed against PF4 (found on platelets and the vascular endothelium) complexed with heparin. Once this complex forms, the Fc region on the platelet is captured by the Fc region on adjacent platelets, perpetuating platelet aggregation.45 Thrombocytopenia occurs when IgG-coated platelets are removed by the reticuloendothelial system and consumed at sites of thrombosis.46
The time from vaccine to symptom onset is 5 to 16 days following the ChAdOx1 vaccine.35,36 Patients had no previous exposure to heparin, suggesting that the anti-PF4 (antibodies directed against platelet factor 4) are a novel humoral response to the vaccine. One possible explanation may be the complexing of nucleic acid contained in the vaccine to PF4.35 Importantly, the incidence rate of VTE as part of this idiosyncratic response to coronavirus vaccines remains low, with extremely rare thrombosis occurring in the cortical veins and dural sinuses (Figure 2). The novelty of this discovery suggests these events may be more common than reported (Figure 3).
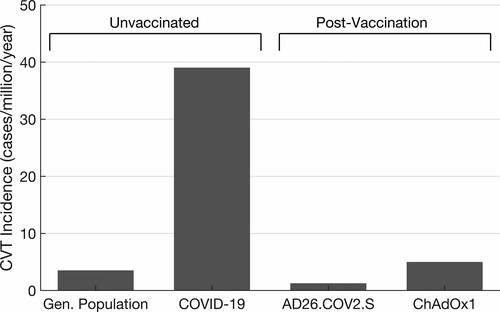
Figure 3. Annualized incidence rates for cerebral vein thrombosis (CVT). COVID-19 indicates coronavirus disease 2019.
Diagnostic Evaluation of CVT and VITT
A high index of suspicion is important in the detection of CVT with VITT for patients who present within 4 weeks of adenovirus-mediated vaccination. In patients with new severe headache, subacute encephalopathy, visual loss, seizure, or focal neurological deficit, a complete blood count and head computed tomography with venography with or without angiography or magnetic resonance imaging with a venogram is recommended.47 If either the platelet count is <150 000/µL or the neuroimaging is suggestive of CVT, the patient will be tested for anti-PF4, preferably an anti-PF4 ELISA.48 Patients with thrombocytopenia should be verified for platelet clumping and recent heparin exposure as alternative mechanisms to explain the low platelets.
Empirical treatment should not be delayed while results are pending. Consultation with a physician with expertise in thrombosis is recommended by the American Heart Association/American Stroke Association to assist in antithrombotic selection and management of CVT49 (with or without VITT) and may aid in the evaluation for alternative causes of consumptive thrombocytopenia. We recommend testing for SARS-CoV-2 by nasopharyngeal polymerase chain reaction, as present vaccines are not fully protective against viral infection, and as COVID-19 has been associated with a low but significant risk of CVT.25 Other causes and risk factors of CVT ought to be considered (Figure 4).50
Management of CVT With VITT
Given the similar mechanism of anti-PF4–induced platelet activation between VITT and HITT, experts recommend that treatment of VITT parallels the treatment of HITT (Figure 4).47
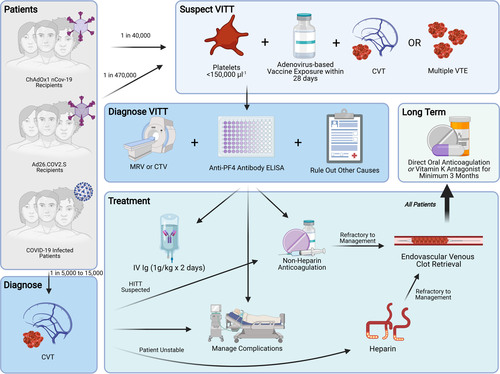
Figure 4. Management of vaccine-induced immune thrombotic thrombocytopenia (VITT). Note that the 1 in 40 000 risk of cerebral vein thrombosis (CVT) following ChAdOx1 vaccination is derived from the estimate by Pottegård et al31 regarding excess event rates rather than laboratory confirmed VITT in CVT. For these excess events, VITT should be suspected. Image generated using Biorender.com. COVID-19 indicates coronavirus disease 2019; CTV, computed tomography venogram; HITT heparin-induced thrombotic thrombocytopenia; MRV magnetic resonance venogram; PF4 platelet factor 4, IVIg intravenous immunoglobulin; and VTE, venous thromboembolism.
Pharmacological Intervention for Presumed VITT
Empirical interventions should target both the autoimmune and thrombotic sequela. As platelet activation via autoantibody formation is thought to be the principal mediator of thrombosis, IVIg (intravenous immunoglobulin) 1g/kg daily for 2 days is recommended,37 although the 2018 Guidelines from the American Society of Hematology do not support its routine use in the related HITT syndrome.51 Potential adverse effects from IVIg including headache, flushing, and aseptic meningitis should be weighed with its potential benefits. High-dose corticosteroids, plasma exchange, and fibrinogen substitution may be considered for severe thrombocytopenia.52
Although there is no evidence that administering heparin for CVT related to VITT is harmful, nonheparin-based intravenous anticoagulants (argatroban, bivalirudin, fondaparinux) are considered due to the presence of anti-PF4 antibodies and overlapping mechanisms with HITT. Oral anticoagulants (rivaroxaban, apixaban, dabigatran) may be considered after clinical stabilization. Anticoagulation with a direct oral anticoagulant or with a vitamin K antagonist should continue for 3 to 6 months or until radiographic resolution of the CVT.49 The American Heart Association/American Stroke Association guidelines recommend anticoagulation be initiated or continued in the presence of hemorrhagic venous congestion,49 although this remains debated.53 In these cases, the associated edema and hematoma size may warrant decompressive hemicraniectomy or hematoma evacuation to prevent fatal herniation. Alternatively, such patients might benefit from endovascular thrombectomy.
Platelet transfusions are not recommended for thrombocytopenia in VITT37,48 unless a life-threatening hemorrhage is present,48 as in HITT guidelines.51 Furthermore, due to the unique mechanism of intracranial hemorrhage in CVT from venous congestion, CVT-associated hemorrhage is unlikely to stabilize with platelet transfusion. Restoration of venous outflow in the form of therapeutic anticoagulation or endovascular intervention is critical to reduce local venous congestion and intracranial pressure, which may prevent edema formation and hematoma expansion.
It is unclear, but heparin (unfractionated and low-molecular weight heparin) may worsen symptoms of VITT due to the mechanistic overlap with HITT. Between 2 previously published series of VITT,35,36 heparin was administered to 11 of 16 patients, with 5 early fatalities. Based on limited data, it is unclear if this mortality rate represents the natural history of the condition or if it reflects a complication of traditional interventions for venous thrombosis. That said, increasing platelet counts were observed in these patients who received concomitant prednisolone and IVIg without evidence of recurrent or increased thrombosis.
Endovascular Retrieval
In patients with deteriorating mental status, endovascular thrombectomy can be considered according to American Heart Association/American Stroke Association guidelines (Class IIb, Level of Evidence C).49,54 However, the benefit of thrombectomy in CVT over medical management was not demonstrated in the recent TO-ACT randomized clinical trial (Thrombolysis or Anticoagulation for Cerebral Venous Thrombosis).55 Because TO-ACT terminated early due to futility, it is possible that thrombectomy may benefit some patients with refractory CVT.
In a patient undergoing venous thrombectomy, if the patient is confirmed or suspected of having VITT, alternatives to heparinized saline flushes (eg, bivalirudin56) may be considered.
COVID-19 Vaccine Paradigms and Challenges
Challenges exist in evaluating the risks and benefits of the continued use of the ChAdOx1 and Ad26.COV2.S vaccines. In cases of potentially life-threatening medical conditions, treatments that carry significant risks remain acceptable. Conversely, the risks involved in the prevention of a medical condition are far less tolerable. Vaccination against COVID-19 using these 2 vaccines carries the risk of a severe complication with a very low incidence (VITT). It is presently unknown what factors, if any, influence the risk of VITT. All vaccines currently being administered in the United States and Europe are available through emergency use decrees rather than through a typical approval process. Such decrees are granted based upon interim analysis of data that may not fully capture the side effect profiles of the intervention. In countries with widespread transmission of SARS-CoV-2, both the World Health Organization and the EMA have suggested that the benefits of vaccination far outweigh the risk of VITT. Shared decision-making and informing the patient of the very low risk of VITT are recommended to clinicians before administration of an adenovirus-based vaccination.
Use of the ChAdOx1 vaccine was resumed in many European countries on March 18, 2021, following a declaration by the EMA. Denmark announced on April 14, that the ChAdOx1 vaccine would not be used following 2 cases of VITT in 140 000 vaccinations. On April 20, 2021, Johnson & Johnson announced their intent to continue administering the Ad26.COV2.S vaccine in Europe following an EMA Pharmacovigilance Risk Assessment Committee report. On April 23, the CDC and Food and Drug Administration recommended the resumption of the Ad26.COV2.S vaccine in the United States based upon a favorable risk-benefit analysis.57
A booster vaccine or annual inoculations may be needed to protect from COVID-19 and emerging variants. In patients who developed VITT from an adenovirus-mediated vaccine, we recommend switching to an mRNA-based vaccine if a second dose is needed.58 In the absence of a VITT related event with the first vaccine, repeat immunization with the previous vaccine type is recommended by the CDC.59
Gaps in Knowledge
The mechanism by which PF4 antibodies develop after adenovirus vaccine exposure remains unknown. It is not known whether VITT has a predilection for the cerebral venous sinuses or the splanchnic bed, compared with more common locations for VTE (such as lungs or legs). Based on population data, it is unknown why VITT has a predisposition for venous rather than arterial thromboembolism.31
Although a rare event, it is unclear why there is a skew towards this affliction in young women, although the United Kingdom VITT series reported 40% of patients were men, and the age extended to a patient in their seventies.37 Predicting VITT will be challenging. In patients who are at higher risk for developing VTE (eg, family history, hypercoagulable state, oral contraceptives, prior VTE), it is not known whether they are at risk of developing VITT. However, when alternative vaccines may be available, a preferential strategy to offer these patients mRNA vaccines may be advised.
Conclusions
The consequences of the COVID-19 pandemic cannot be understated. Global health measures aimed at reducing the spread of the virus, including community education, universal masking, social distancing, and handwashing, have only temporized the pandemic. To date, the best treatment of COVID-19 involves supportive care and management of the para-infectious complications, including thrombotic events. The emergence of more contagious variants may accelerate the spread of the virus.
The optimal means of reducing the spread of SARS-CoV-2 remains mass pharmacological prophylaxis and establishment of herd immunity. The COVID-19 vaccines have proven efficacy against infection with SARS-CoV-2, severe COVID-19, and they have proven efficacy against emerging SARS-CoV-2 variants. Of the rarer complications of these vaccines, an autoimmune-mediated thrombotic thrombocytopenia is likely to be increasingly recognized. To date, this complication is unique to adenoviral coronavirus vaccines and preferentially affects young and middle-aged women without preexisting conditions. Early recognition, diagnosis, and treatment of VITT tailored to its pathophysiology (ie, suppressing the antibody response with IVIg, avoiding platelet transfusions, reducing thrombus burden with nonheparin-based anticoagulants) may improve the current high mortality rate of VITT (20%–30%).32,58
Although the morbidity and mortality of VITT are highly concerning, it remains a rare event. The patient-level and societal benefits of vaccination vastly exceed the known risks of available coronavirus vaccines. We strongly encourage healthcare providers to continue recommending any available and approved COVID-19 vaccine for eligible persons because the risks of COVID-19 are greater than the risks of CVT. However, if >1 vaccine option is available, it may be reasonable to consider a nonadenoviral vaccine for women under the age of 50 years. In light of the United Kingdom VITT data,37 it may be reasonable to extend consideration of nonadenoviral vaccines for young men as well. A high index of suspicion for VITT is important as early diagnosis can impact patient management and outcome.
As we learn more about VITT, recommendations for management are likely to change. It would be prudent to consult a local hematologist in the event of any suspicious event and apply updated guidance statements from the CDC, World Health Organization, or other hematologic society into the care of patients with this rare complication. Providers are reminded to report new cases to the Vaccine Adverse Event Reporting system (https://vaers.hhs.gov/) to better understand the true incidence of this entity and other complications.
Sources of Funding = None.
Supplemental Materials
Online Table I
Disclosures Dr Siegler reports consulting fees from AstraZeneca and Ceribell, unrelated to the present work. Dr Yaghi reports unfunded research collaboration with Medtronic. Dr Coutinho reports grants from Boehringer and Bayer, unrelated to this work. Dr Nguyen reports research support from Medtronic and the Society of Vascular and Interventional Neurology, unrelated to this work.
Footnotes
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time.Lancet Infect Dis. 2020; 20:533–534. doi: 10.1016/S1473-3099(20)30120-1CrossrefMedlineGoogle Scholar
- 2.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, ; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 Infection.J Am Coll Cardiol. 2020; 76:533–546. doi: 10.1016/j.jacc.2020.06.007CrossrefMedlineGoogle Scholar
- 3.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis.Res Pract Thromb Haemost. 2020; 4:1178–1191.CrossrefMedlineGoogle Scholar
- 4.Siegler JE, Cardona P, Arenillas JF, Talavera B, Guillen AN, Chavarría-Miranda A, de Lera M, Khandelwal P, Bach I, Patel P, . Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 Multinational Registry.Int J Stroke. 2020; 16:437–447. doi: 10.1177/1747493020959216.Google Scholar
- 5.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, . SARS-CoV-2 and stroke in a New York healthcare system.Stroke. 2020; 51:2002–2011. doi: 10.1161/STROKEAHA.120.030335LinkGoogle Scholar
- 6.Nogueira RG, Abdalkader M, Qureshi MM, Frankel MR, Mansour OY, Yamagami H, Qiu Z, Farhoudi M, Siegler JE, Yaghi S, . Global impact of COVID-19 on stroke care.Int J Stroke. 2021; 96:e2824–e2838. doi: 10.1212/WNL.0000000000011885Google Scholar
- 7.Nogueira RG, Qureshi MM, Abdalkader M, Martins SO, Yamagami H, Qiu Z, Mansour OY, Sathya A, Czlonkowska A, Tsivgoulis G, ; SVIN COVID-19 Global Stroke Registry; SVIN COVID-19 Global Stroke Registry. Global impact of COVID-19 on stroke care and IV thrombolysis.Neurology. 2021; 96:e2824–e2838. doi: 10.1212/WNL.0000000000011885Google Scholar
- 8.Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study.Lancet Respir Med. 2021; 9:251–259. doi: 10.1016/S2213-2600(20)30527-0Google Scholar
- 9.Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic.Lancet. 2021; 397:952–954. doi: 10.1016/S0140-6736(21)00370-6Google Scholar
- 10.Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, Slayton RB, Tong S, Silk BJ, Armstrong GL, . Emergence of SARS-CoV-2 b. 1.1. 7 lineage—united states, december 29, 2020–january 12, 2021.MMWR Surveill Summ. 2021; 70:95.Google Scholar
- 11.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, . Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B. 1.351 variant in South Africa.New Engl J Med. 2021. [published online March 16, 2021]. https://www.nejm.org/doi/full/10.1056/nejmoa2102214#:~:text=Interim%20results%20from%20South%20Africa,1.351%20variant.&text=The%20Ad26. Accessed Apr 25, 2021.Google Scholar
- 12.Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant.BMJ. 2021; 372:n296. doi: 10.1136/bmj.n296Google Scholar
- 13.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, Blane B, Bonsall D, Cicconi P, Charlton S, ; COVID-19 Genomics UK consortium; AMPHEUS Project; Oxford COVID-19 Vaccine Trial Group. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial.Lancet. 2021; 397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0Google Scholar
- 14.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, ; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2020; 383:2603–2615. doi: 10.1056/NEJMoa2034577CrossrefMedlineGoogle Scholar
- 15.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK.Lancet. 2021; 397:99–111. doi: 10.1016/S0140-6736(20)32661-1CrossrefMedlineGoogle Scholar
- 16.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, . Interim results of a Phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine.N Engl J Med. 2021; 384:1824–1835. doi: 10.1056/NEJMoa2034201Google Scholar
- 17.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, ; Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia.Lancet. 2021; 397:671–681. doi: 10.1016/S0140-6736(21)00234-8Google Scholar
- 18.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, . Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet. 2020; 396:479–488. doi: 10.1016/S0140-6736(20)31605-6Google Scholar
- 19.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine.N Engl J Med. 2021; 384:403–416. doi: 10.1056/NEJMoa2035389CrossrefMedlineGoogle Scholar
- 20.Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19.Am J Emerg Med. 2021; 39:213–218. doi: 10.1016/j.ajem.2020.09.065Google Scholar
- 21.Ma A, Kase CS, Shoamanesh A, Abdalkader M, Pikula A, Sathya A, Catanese L, Ellis AT, Nguyen TN. Stroke and thromboprophylaxis in the era of COVID-19.J Stroke Cerebrovasc Dis. 2021; 30:105392. doi: 10.1016/j.jstrokecerebrovasdis.2020.105392Google Scholar
- 22.Flumignan RLG, de Sá Tinôco JD, Pascoal PIF, Areias LL, Cossi MS, Fernandes MI, Costa IKF, Souza L, Matar CF, Tendal B, . Prophylactic anticoagulants for people hospitalised with COVID-19.Cochrane Database Syst. Rev. 2020; 10:CD013739. doi: 10.1002/14651858.CD013739Google Scholar
- 23.NIH ACTIV Trial of Blood Thinners Pauses Enrollment of Critically ill COVID-19 Patients.2020. https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients. Accessed Apr 25, 2021.Google Scholar
- 24.Zarychanski R; The REMAP-CAP. ACTIV-4a, ATTACC Investigators. Therapeutic anticoagulation in critically ill patients with Covid-19 – preliminary report.bioRxiv. 2021. Preprint posted online March 12, 2021. doi: 10.1101/2021.03.10.21252749Google Scholar
- 25.Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, Tiu VE, Jillella DV, Mansour OY, Vera V, . Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature.J Stroke Cerebrovasc Dis. 2021; 30:105733. doi: 10.1016/j.jstrokecerebrovasdis.2021.105733CrossrefMedlineGoogle Scholar
- 26.Mowla A, Shakibajahromi B, Shahjouei S, Borhani-Haghighi A, Rahimian N, Baharvahdat H, Naderi S, Khorvash F, Altafi D, Ebrahimzadeh SA, . Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series.J Neurol Sci. 2020; 419:117183. doi: 10.1016/j.jns.2020.117183CrossrefMedlineGoogle Scholar
- 27.Al-Mufti F, Amuluru K, Sahni R, Bekelis K, Karimi R, Ogulnick J, Cooper J, Overby P, Nuoman R, Tiwari A, . Cerebral venous thrombosis in COVID-19: a New York Metropolitan Cohort Study [published online April 22, 2021].AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A7134. http://www.ajnr.org/content/early/2021/04/22/ajnr.A7134. Accessed April 25, 2021.Google Scholar
- 28.Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, Aguiar De Sousa D, Sellner J, Zini A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis [published online January 11, 2021].Eur J Neurol. doi: 10.1111/ene.14727. https://onlinelibrary.wiley.com/doi/full/10.1111/ene.14727. Accessed April 25, 2021.Google Scholar
- 29.Hinduja A, Nalleballe K, Onteddu S, Kovvuru S, Hussein O. Impact of cerebral venous sinus thrombosis associated with COVID-19.J Neurol Sci. 2021; 425:117448. doi: 10.1016/j.jns.2021.117448Google Scholar
- 30.Pinho AC. AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets.2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. Accessed May 16, 2021.Google Scholar
- 31.Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL, . Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study.BMJ. 2021; 373:n1114. doi: 10.1136/bmj.n1114Google Scholar
- 32.Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine – United States, March-April 2021.MMWR Morb Mortal Wkly Rep. 2021; 70:680–684. doi: 10.15585/mmwr.mm7018e2Google Scholar
- 33.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF, . US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021.JAMA. 2021; 325:2448–2456. doi: 10.1001/jama.2021.7517CrossrefMedlineGoogle Scholar
- 34.Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine astrazeneca” exposure.J Clin Med Res. 2021; 10:1599. doi: 10.3390/jcm10081599Google Scholar
- 35.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination.N Engl J Med. 2021; 384:2092–2101. doi: 10.1056/NEJMoa2104840CrossrefMedlineGoogle Scholar
- 36.Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, . Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination.N Engl J Med. 2021; 384:2124–2130. doi: 10.1056/NEJMoa2104882CrossrefMedlineGoogle Scholar
- 37.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination.N Engl J Med. 2021; 384:2202–2211. doi: 10.1056/NEJMoa2105385CrossrefMedlineGoogle Scholar
- 38.Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, Sztriha LK. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination – A report of two UK cases.Brain Behav Immun. 2021; 95:514–517. doi: 10.1016/j.bbi.2021.04.006Google Scholar
- 39.Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19.Crit Care. 2021; 25:137. doi: 10.1186/s13054-021-03572-yGoogle Scholar
- 40.Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag FG, Weißenborn K, Höglinger GU, Maasoumy B, . Prothrombotic immune thrombocytopenia after COVID-19 vaccine [published online April 28, 2021]. Blood. doi: 10.1182/blood.2021011958. https://ashpublications.org/blood/article/doi/10.1182/blood.2021011958/475845/Prothrombotic-immune-thrombocytopenia-after-COVID. Accessed May 16, 2021.Google Scholar
- 41.ACIP April 14, 2021 Presentation Slides.2021. [published Apr 14, 2021] https://www.cdc.gov/vaccines/acip/meetings/slides-2021-04.html. Accessed May 16, 2021.Google Scholar
- 42.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting.N Engl J Med. 2021; 384:1412–1423. doi: 10.1056/NEJMoa2101765CrossrefMedlineGoogle Scholar
- 43.Pollard AJ, Launay O, Lelievre JD, Lacabaratz C, Grande S, Goldstein N, Robinson C, Gaddah A, Bockstal V, Wiedemann A, ; EBOVAC2 EBL2001 study group. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2021; 21:493–506. doi: 10.1016/S1473-3099(20)30476-XGoogle Scholar
- 44.Hursting MJ, Pai PJ, McCracken JE, Hwang F, Suvarna S, Lokhnygina Y, Bandarenko N, Arepally GM. Platelet factor 4/heparin antibodies in blood bank donors.Am J Clin Pathol. 2010; 134:774–780. doi: 10.1309/AJCPG0MNR5NGKNFXGoogle Scholar
- 45.Rollin J, Pouplard C, Gruel Y. Risk factors for heparin-induced thrombocytopenia: focus on Fcγ receptors.Thromb Haemost. 2016; 116:799–805. doi: 10.1160/TH16-02-0109Google Scholar
- 46.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia.N Engl J Med. 1987; 316:581–589. doi: 10.1056/NEJM198703053161004CrossrefMedlineGoogle Scholar
- 47.American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced thrombotic thrombocytopenia.Stroke. 2021; 52:2478–2482. doi: 10.1161/STROKEAHA.121.035564Google Scholar
- 48.Brooks JT, Su JR, Connors JM, Kreuziger LB. Triage and Treatment of Patients with Potential Vaccine-induced Immune Thrombocytopenia (VITT) Post J&J Covid-19 vaccination.2021. https://www.zoomgov.com/rec/play/Yr3V913G4fZZnvKXa4Jmqe_dfJ-Pt-lseD8jPLIYzGH8U2ikES7183Tx_IgYqLAor7iehmzCZNx3uWru.nivW996P0qKRnGrz?continueMode=true. Accessed April 25, 2021.Google Scholar
- 49.Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association.Stroke. 2011; 42:1158–1192. doi: 10.1161/STR.0b013e31820a8364LinkGoogle Scholar
- 50.McBane RD, Tafur A, Wysokinski WE. Acquired and congenital risk factors associated with cerebral venous sinus thrombosis.Thromb Res. 2010; 126:81–87. doi: 10.1016/j.thromres.2010.04.015Google Scholar
- 51.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, . American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia.Blood Adv. 2018; 2:3360–3392. doi: 10.1182/bloodadvances.2018024489CrossrefMedlineGoogle Scholar
- 52.International Society on Thrombosis and Haemostasis. ISTH Interim Guidance for the Diagnosis and Treatment on VaccineInduced Immune Thrombotic Thrombocytopenia.2021. [published online Apr 20, 2021] https://cdn.ymaws.com/www.isth.org/resource/resmgr/ISTH_VITT_Guidance_2.pdf. Accessed April 25, 2021.Google Scholar
- 53.Pizzi MA, Alejos DA, Siegel JL, Kim BY, Miller DA, Freeman WD. Cerebral venous thrombosis associated with intracranial hemorrhage and timing of anticoagulation after hemicraniectomy.J Stroke Cerebrovasc Dis. 2016; 25:2312–2316. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.025Google Scholar
- 54.Eskey CJ, Meyers PM, Nguyen TN, Ansari SA, Jayaraman M, McDougall CG, DeMarco JK, Gray WA, Hess DC, Higashida RT, ; American Heart Association Council on Cardiovascular Radiology and Intervention and Stroke Council. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association.Circulation. 2018; 137:e661–e689. doi: 10.1161/CIR.0000000000000567LinkGoogle Scholar
- 55.Coutinho JM, Zuurbier SM, Bousser MG, Ji X, Canhão P, Roos YB, Crassard I, Nunes AP, Uyttenboogaart M, Chen J, ; TO-ACT investigators. Effect of endovascular treatment with medical management vs standard care on severe cerebral venous thrombosis: the TO-ACT Randomized Clinical Trial.JAMA Neurol. 2020; 77:966–973. doi: 10.1001/jamaneurol.2020.1022Google Scholar
- 56.Hassan AE, Memon MZ, Georgiadis AL, Vazquez G, Suri MF, Qureshi AI. Safety and tolerability of high-intensity anticoagulation with bivalirudin during neuroendovascular procedures.Neurocrit Care. 2011; 15:96–100. doi: 10.1007/s12028-010-9421-7Google Scholar
- 57.Center for Disease Control. FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review [Internet]. [published online Apr 23, 2021].https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough. Accessed April 25, 2021.Google Scholar
- 58.Coronavirus vaccine – weekly summary of Yellow Card reporting. [published update online May 5, 2021].https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed May 16, 2021.Google Scholar
- 59.Centers for Disease Control. Vaccines for COVID-19.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html. Accessed April 25, 2021.Google Scholar
source: James E. Siegler,Piers Klein,Shadi Yaghi,Nicholas Vigilante,Mohamad Abdalkader,Jonathan M. Coutinho,Feras Abdul Khalek and Thanh N. Nguyen
Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study
Summary
Background
Methods
Findings
Interpretation
Funding
Introduction
Research in context
Methods
Study design and participants
), clinical features, laboratory results, radiological findings, and treatments given, with death or dependency (modified Rankin score13
3–6) at the end of hospital admission as the primary outcome. Data were checked centrally for omissions, duplications, or inconsistencies, and data queries were sent back to the submitting clinicians until these were resolved. Case report forms were received between April 1 and May 20, 2021. The UK Health Research Authority confirmed that this surveillance study, using routine patient data in anonymised form, could proceed without the need for patient consent or review by an ethics committee.
Defining VITT-associated cerebral venous thrombosis
These criteria are referred to as the starting criteria (different from the proposed criteria in the panel). Before proceeding with any comparisons between groups, we examined the frequency distributions of the minimum platelet count and maximum D-dimers recorded during admission across the whole study population, to confirm the appropriateness of these diagnostic thresholds in a population of patients with cerebral venous thrombosis.
Anti-PF4 antibody assays
Statistical analysis
using the one-sample Wilcoxon signed rank test. All other continuous variables were compared using the Mann-Whitney U test.
Role of the funding source
Results
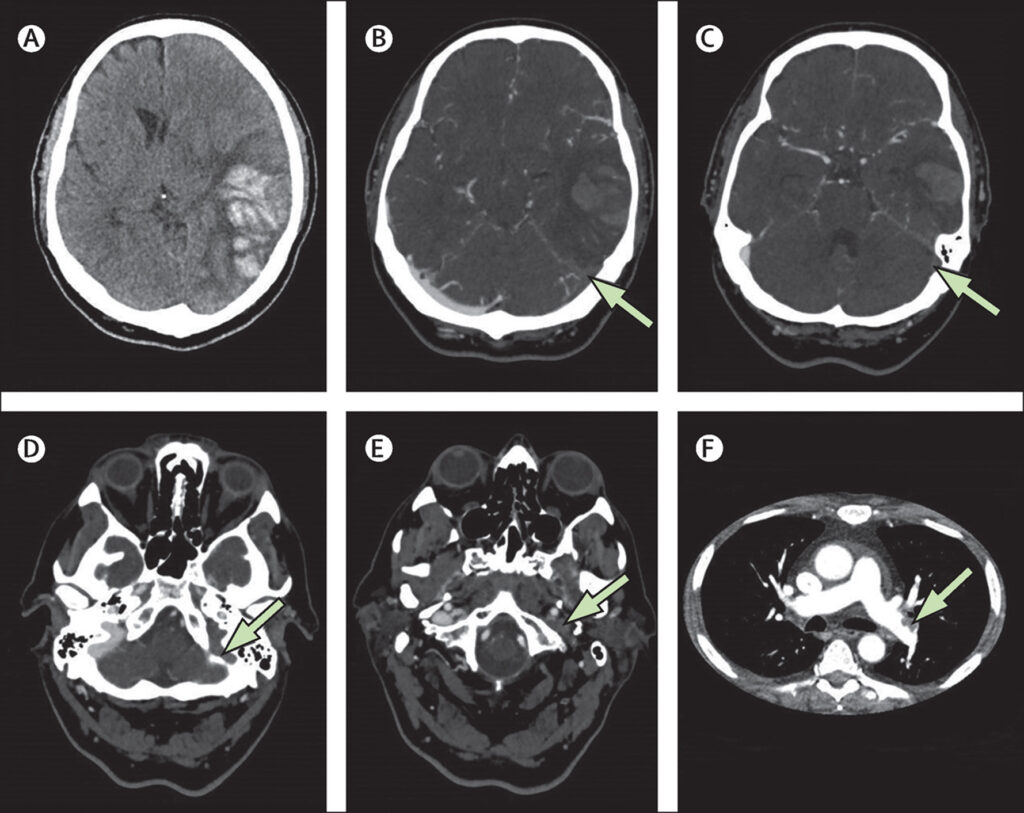
Between April 1 and May 20, 2021, we received data on 99 patients from collaborators in 43 hospitals across the UK. Four patients were excluded because they did not have definitive evidence of cerebral venous thrombosis on imaging (appendix p 9). In 83 (87%) of 95 patients, the modality on which cerebral venous thrombosis was shown was CT venography (figure 1). The lowest platelet count during admission was available for all 95 patients and the highest D-dimer was available in 62 (89%) of 70 patients with VITT and 20 (80%) of 25 patients without VITT.
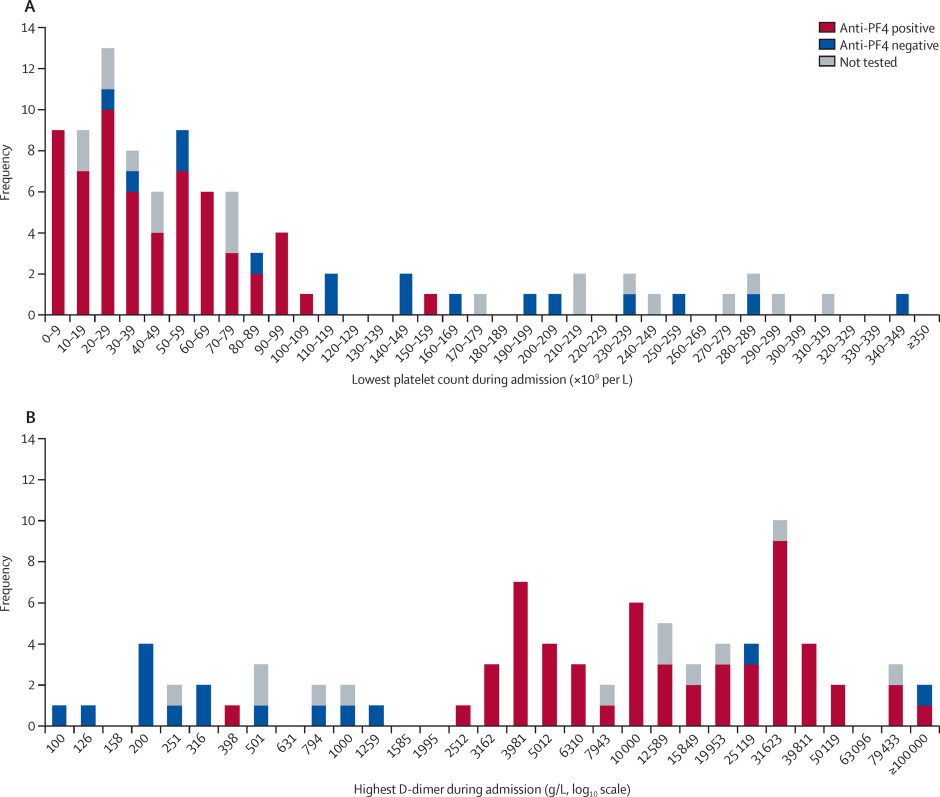
The median time interval between vaccination and cerebral venous thrombosis symptom onset was 9 days (IQR 7–12) in patients with VITT and 11 days (6–21) in those without VITT (table 1; appendix p 10). One patient with VITT developed clumsiness of the left arm 40 days after the first and only dose of ChAdOx1 vaccine, the first manifestation of a cortical vein thrombosis. However, the patient had developed a deep vein thrombosis, their first manifestation of VITT, 21 days after vaccination. The deep vein thrombosis was initially treated with tinzaparin, but the patient was found to be thrombocytopenic before this treatment was started. This patient was the only individual in the whole study to receive any form of heparin within the 2 weeks preceding the cerebral venous thrombosis.
| VITT (n=70) | Non-VITT (n=25) | p value (VITT vs non-VITT) | ISCVT cohort (n=624) | p value (VITT vs ISCVT) | ||
|---|---|---|---|---|---|---|
| Age, years | 47 (32–55) | 57 (41–62) | 0·0045 | 37 | 0·0001 | |
| Sex | 0·31 | 0·0007 | ||||
| Female | 39 (56%) | 11 (44%) | 465 (75%) | |||
| Male | 31 (44%) | 14 (56%) | .. | 159 (25%) | ||
| Ethnicity | ||||||
| White | 61 (87%) | 21 (84%) | 0·74 | 550/621 (89%) | 0·72 | |
| Asian | 7 (10%) | 2 (8%) | 1·00 | 21/621 (3%) | 0·017 | |
| Black | 0 | 1 (4%) | 0·26 | 31/621 (5%) | 0·063 | |
| Other or mixed | 2 (3%) | 1 (4%) | 1·00 | 19/621 (3%) | 1·00 | |
| Vaccine details | ||||||
| Proportion given AstraZeneca vaccine | 70 (100%) | 21 (84%) | 0·0040 | .. | .. | |
| Time from vaccine to cerebral venous thrombosis, days | 9 (7–12) | 11 (6–21) | 0·10 | .. | .. | |
| Venous risk factors | ||||||
| Patients with no venous risk factors | 46 (66%) | 11 (44%) | 0·057 | .. | .. | |
| Patients with no ISCVT risk factors | 61 (87%) | 20 (80%) | 0·51 | 78 (13%) | <0·0001 | |
| Fibrinogen, g/L | 2·0 (1·3–2·8)
*
|
3·3 (2·9–4·1)
†
|
0·0001 | .. | .. | |
| Prothrombin time, s | 13·0 (11·9–14·8)
‡
|
11·5 (10·8–12·6)
§
|
0·0005 | .. | .. | |
| Activated partial thrombloplastin time, s | 28·8 (25·1–34·8)
¶
|
26·9 (24·4–32·7)
‖
|
0·030 | .. | .. | |
| Anti-platelet factor 4 antibodies | ||||||
| Positive on ELISA | 56/58 (97%) | 2/16 (13%) | <0·0001 | .. | .. | |
| Positive on Acustar HIT-IgG assay | 3/13 (23%) | 0 | 0·52 | .. | .. | |
the step-change in frequency above age 45 years was no longer apparent (appendix p 10).
Patients with VITT were significantly younger than patients who did not have VITT (table 1). All 70 cases of VITT-associated cerebral venous thrombosis occurred after a first dose of the ChAdOx1 (Oxford–AstraZeneca) vaccine, compared with 21 (85%) of 25 patients with non-VITT cerebral venous thrombosis, in whom the remaining four patients had been given their first dose (three patients) or second dose (one patient) of BNT162b2 (Pfizer–BioNTech) vaccine. The clinical features of cerebral venous thrombosis were similar in the VITT and non-VITT groups (appendix p 4).
We compared the modified Rankin scale13 at discharge for patients with VITT compared with the non-VITT group (figure 3A) and the ISCVT cohort (figure 3B). The primary outcome, death or dependency on others for care (modified Rankin score 3–6), was significantly more common in patients with VITT-associated cerebral venous thrombosis (33 [47%] of 70 patients) than in patients without VITT (four [16%] of 25 patients; p=0·0061). More patients died during admission in the VITT-associated cerebral venous thrombosis group (20 [29%] of 70 patients) than in the non-VITT group (one [4%] of 25 patients; p=0·011). Low Glasgow Coma Scale15 on admission and cerebral haemorrhage were the strongest predictors of death or dependency, as expected in patients with cerebral venous thrombosis (appendix p 6).12
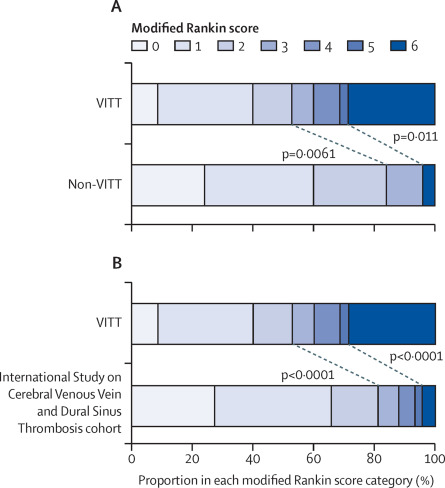
(A) Comparison of the outcomes from cerebral venous thrombosis in patients with VITT versus patients without VITT. (B) Comparison between VITT-associated cerebral venous thrombosis and historical data from the International Study on Cerebral Vein and Dural Sinus Thrombosis cohort. Each horizontal bar represents the percentage of patients in each modified Rankin scale category, which varies from 0 (no symptoms) through to 5 (severe disability). 6 represents death during this hospital admission. Diagonal lines and p values show comparisons of death and dependency (modified Rankin score 3–6) or death (modified Rankin score 6). VITT=vaccine-induced immune thrombotic thrombocytopenia.
The proportion of patients with VITT who had died or were dependent on others for their care at the end of admission was significantly lower in those given non-heparin parenteral anticoagulation (18 [36%] of 50 patients) compared with those who were not (15 [75%] of 20 patients; p=0·0031), in those who were given a direct oral anticoagulant (four [18%] of 22 patients) compared with those who were not (29 [60%] of 48 patients; p=0·0016), and in those who were given intravenous immunoglobulin (22 [40%] of 55 patients) compared with those who were not (11 [73%] of 15 patients; p=0·022; table 2).
| Patients treated or not treated | Patients who had died or were dependent | p value | ||
|---|---|---|---|---|
| Pharmacological | ||||
| Any anticoagulation | .. | .. | 0·0047 | |
| Yes | 60 | 24 (40%) | .. | |
| No | 10 | 9 (90%) | .. | |
| Heparin or low-molecular-weight heparin | .. | .. | 1·0 | |
| Yes | 16 | 8 (50%) | .. | |
| No | 54 | 25 (46%) | .. | |
| Non-heparin parenteral anticoagulant | .. | .. | 0·0031 | |
| Yes | 50 | 18 (36%) | .. | |
| No | 20 | 15 (75%) | .. | |
| Direct oral anticoagulant | .. | .. | 0·0016 | |
| Yes | 22 | 4 (18%) | .. | |
| No | 48 | 29 (60%) | .. | |
| Corticosteroid | .. | .. | 0·27 | |
| Yes | 51 | 22 (43%) | .. | |
| No | 19 | 11 (58%) | .. | |
| Anticonvulsant | .. | .. | 0·71 | |
| Yes | 26 | 13 (50%) | .. | |
| No | 44 | 24 (55%) | .. | |
| Fibrinogen replacement | .. | .. | 1·00 | |
| Yes | 15 | 7 (47%) | .. | |
| No | 55 | 26 (47%) | .. | |
| Intravenous immunoglobulin | .. | .. | 0·022 | |
| Yes | 55 | 22 (40%) | .. | |
| No | 15 | 11 (73%) | .. | |
| Plasma exchange | .. | .. | 0·78 | |
| Yes | 16 | 7 (44%) | .. | |
| No | 54 | 26 (48%) | .. | |
| Platelet transfusion | .. | .. | <0·0001 | |
| Yes | 25 | 21 (84%) | .. | |
| No | 45 | 12 (27%) | .. | |
| Invasive | ||||
| Endovascular management | .. | .. | 0·73 | |
| Yes | 9 | 5 (56%) | .. | |
| No | 61 | 28 (46%) | .. | |
| Intracranial pressure monitor | .. | .. | <0·0001 | |
| Yes | 13 | 13 (100%) | .. | |
| No | 57 | 20 (35%) | .. | |
| Decompressive hemicraniectomy | .. | .. | <0·0001 | |
| Yes | 13 | 13 (100%) | .. | |
| No | 57 | 20 (35%) | .. | |
Discussion
The ratio of patients with VITT to patients without VITT was 2·8:1, as expected from the estimated incidence of VITT-associated cerebral venous thrombosis in individuals receiving a first dose of the ChAdOx2 vaccine (12·3 per million
) and the expected background incidence of cerebral venous thrombosis in the same subpopulation during the 4-month study period (4·4 per million
), suggesting that cerebral venous thrombosis was probably unrelated to vaccination in most or all of our non-VITT cases and that there was no significant bias towards reporting of VITT cases.
A normal platelet count (conventionally ≥150 × 109 per L) is regarded as ruling out VITT in existing peer-reviewed published guidelines,
,
but adopting a platelet count threshold of less than 150 × 109 per L as a criterion for VITT-associated cerebral venous thrombosis in the present study could have been a weakness. First, defining thrombocytopenia as a fall to less than 50% of a known baseline platelet count is recommended in the analogous condition of heparin-induced thrombocytopenia.
Second, patient B (appendix p 3), who was excluded from our VITT group because her platelet count did not fall below 150 × 109 per L, was treated as having VITT because of positive anti-PF4 antibodies and very high D-dimer of 4985 μg/L. Although we regard thrombocytopenia as the hallmark for VITT, adopting a hard threshold of 150 × 109 per L for defining thrombocytopenia risks excluding patients who have good evidence of VITT.
Aside from the lowest platelet count and highest D-dimer that were used to make the diagnosis of VITT-associated cerebral venous thrombosis, three other features showed a significant association with the diagnosis: anti-PF4 antibodies, fibrinogen, and extracranial venous thromboses. The specificity of anti-PF4 antibodies was probably underestimated in our study, as the only two patients who were positive for the antibody but were classified as non-VITT using current criteria were patients B and C (appendix p 3)—ie, patients with probable VITT who were most likely misclassified. However, patients E and F (appendix p 3) had evidence for VITT but both were negative for anti-PF4 antibodies on two different ELISA assays, suggesting that a negative ELISA result should not be used to define VITT as unlikely
or to cease further investigation,
as is recommended in existing guidelines.
,
These observations lead us to propose a new set of diagnostic criteria for VITT-associated cerebral venous thrombosis (panel). A diagnosis of possible VITT-associated cerebral venous thrombosis will alert clinicians to an urgent need for further investigation for this condition and they are likely to avoid the use of heparins or platelet transfusions if possible. A diagnosis of probable VITT constitutes sufficient evidence to offer a patient full treatment for this condition, including intravenous immunoglobulin or plasma exchange. A definite diagnosis will be useful for defining a population for future research studies on this condition. According to these criteria it is possible to make a diagnosis of probable VITT in patients with a normal platelet count (≥150 × 109 per L), a normal D-dimer, or a negative anti-PF4 antibody test, provided other evidence strongly supports the diagnosis.
Diagnostic criteria for VITT-associated cerebral venous thrombosis
- •
Post-vaccine cerebral venous thrombosis (proven on neuroimaging and with first symptom of venous thrombosis within 28 days of vaccination against COVID-19)
- •
Thrombocytopenia (lowest recorded platelet count <150 × 109 per L or documented platelet count decrease to less than 50% of baseline)
- •
Anti-PF4 antibodies (detected on ELISA or functional assay)
- •
Post-vaccine cerebral venous thrombosis
- •
Either thrombocytopenia or anti-PF4 antibodies
- •
Coagulopathy (D-dimer >2000 μg/L or fibrinogen <2·0 g/L with no other explanation such as severe sepsis, malignancy, or recent trauma or surgery) or extracranial venous thrombosis (clinical or imaging evidence with onset since vaccination against COVID-19)
- •
Post-vaccine cerebral venous thrombosis
- •
Either thrombocytopenia or anti-PF4 antibodiesIn assessing the interval since vaccination, the date of the first symptom of venous thrombosis should be used, even if this was a symptom of an extracranial thrombosis. The retrospective time window within which a pre-cerebral venous thrombosis baseline platelet count can be used to define a fall of greater than 50% has not been defined, as this will depend on what medical events have occurred in the interim.
Anticoagulation and treatment with intravenous immunoglobulin were associated with a lower probability of death or dependency at the end of hospital admission, but this finding is difficult to interpret, as the most unwell patients might have died before these treatments could be offered, biasing the results. Similarly, the association between decompressive hemicraniectomy and poor outcome probably reflects selection of patients with the most severe cerebral venous thrombosis for this invasive procedure. However, the mortality rate of 54% after decompressive hemicraniectomy for VITT-associated cerebral venous thrombosis is high compared with a historical mortality of 16% after this procedure in cerebral venous thrombosis.
The relationship between platelet transfusion and poor outcome in VITT-associated cerebral venous thrombosis appears to support concerns about the safety of this treatment,
but the findings are difficult to interpret; in 12 (48%) of 25 patients offered this treatment, the indication was to support decompressive hemicraniectomy, which was only offered to patients with severe cerebral venous thrombosis.
We present the largest and most detailed study of VITT-associated cerebral venous thrombosis to date, with a well-matched control group consisting of patients presenting to UK hospitals with cerebral venous thrombosis after vaccination against COVID-19 but without evidence of VITT. However, our study has some limitations. The number of patients in each group in our study was small, because of the rarity of these conditions. The study was underpowered for some of the comparisons made between the VITT and non-VITT groups. Although our study will generate important hypotheses for future research, we cannot draw inferences about other populations of patients with cerebral venous thrombosis after COVID-19 vaccination. Comparison of our patients with the much larger historical ISCVT cohort
might have been confounded by the higher age of our patients, attributable to COVID-19 vaccination policy in the UK, rather than to VITT. The median interval between vaccination and symptom onset could be an underestimate; in some cases in which the first symptom of cerebral venous thrombosis was reported as headache, this symptom might initially have been caused by mechanisms other than cerebral venous thrombosis, and also patients with a shorter interval might have been preferentially reported. We were dependent on local radiology reports for interpretation of scans, and on routine clinical observations, laboratory tests, and radiology, which might have led to indication bias. For example, we found only one patient with anti-PF4 antibodies but normal platelets (patient B; appendix p 3), but nine (45%) of 20 patients with normal platelets were not checked for anti-PF4 antibodies, so other cases with this combination might have been missed. We were unable to draw firm conclusions about treatments for VITT-associated cerebral venous thrombosis because we could not control for differences in the baseline characteristics between patients offered or not offered those treatments.
In conclusion, we have described the clinical features of VITT-associated cerebral venous thrombosis in detail, allowing us to propose diagnostic criteria for this condition. We recommend that all patients presenting with cerebral venous thrombosis within 28 days of COVID-19 vaccination should be checked for anti-PF4 antibodies, whatever their platelet count, until there are sufficient data to set an upper limit on the platelet count with which VITT-associated cerebral venous thrombosis might occur. We have shown that VITT-associated cerebral venous thrombosis has poorer outcomes than other forms of cerebral venous thrombosis and our data suggest that non-heparin anticoagulants and immunoglobulin might improve outcomes. However, VITT appears to be a very rare side-effect of vaccination with the ChAdOx1 (Oxford–AstraZeneca) vaccine, the risk of which is likely to be greatly outweighed by the benefit of vaccination against COVID-19 for most people.
Data sharing
Declaration of interests
Supplementary Material
-
Supplementary appendix
References
- 1.
Coronavirus (COVID-19) deaths.
https://ourworldindata.org/covid-deaths
Date accessed: July 22, 2021 - 2.
Coronarivus (COVID-19) vaccinations.
https://ourworldindata.org/covid-vaccinations
Date accessed: July 22, 2021 - 3.
Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination.
N Engl J Med. 2021; 384: 2124-2130
- 4.
Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination.
N Engl J Med. 2021; 384: 2202-2211
- 5.
Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination.
N Engl J Med. 2021; 384: 2092-2101
- 6.
Thrombotic thrombocytopenia after Ad26.COV2.S vaccination.
N Engl J Med. 2021; 384: 1964-1965
- 7.
US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021.
JAMA. 2021; 325: 2448-2456
- 8.
Severe, refractory immune thrombocytopenia occurring after SARS-CoV-2 vaccine.
J Blood Med. 2021; 12: 221-224
- 9.
Purpuric rash and thrombocytopenia after the mRNA-1273 (Moderna) COVID-19 vaccine.
Cureus. 2021; 13e14099
- 10.
Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination.
Am J Hematol. 2021; 96: 534-537
- 11.
Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase.
Eur Respir J. 2021; 582100956
- 12.
Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT).
Stroke. 2004; 35: 664-670
- 13.
Interobserver agreement for the assessment of handicap in stroke patients.
Stroke. 1988; 19: 604-607
- 14.
OpenSAFELY NHS COVID-19 Vaccine Coverage.
https://www.opensafely.org/research/2021/covid-vaccine-coverage
Date accessed: May 12, 2021 - 15.
Assessment of coma and impaired consciousness. A practical scale.
Lancet. 1974; 2: 81-84
- 16.
- 16.
Coronavirus vaccine—weekly summary of yellow card reporting.Date accessed: May 12, 2021
- 17.
The incidence of cerebral venous thrombosis: a cross-sectional study.
Stroke. 2012; 43: 3375-3377
- 18.
Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH.
Hamostaseologie. 2021; 41: 184-189
- 19.
Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology.
J Thromb Haemost. 2021; 19: 1585-1588
- 20.
An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients.
Arch Intern Med. 2003; 163: 2518-2524
- 21.
Role of decompressive craniectomy in the management of cerebral venous sinus thrombosis.
Front Neurol. 2019; 10: 511
- 22.
Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537 913 COVID-19 cases.
EClinicalMedicine. 2021; 39101061
Article info
Publication history
Identification
Copyright
ScienceDirect
Soruces