9 Ways Gut Bacteria And Mental Health, Probiotics And Depression Are Linked
Gut bacteria are key players in your mood and mental health. They can relieve the symptoms of depression, anxiety, and stress, but they might also make them worse.
Your colon is home to trillions of bacterial cells which make up a unique ecosystem called the gut microbiome. As well as allowing nutrients to enter the body and keeping opportunistic pathogens locked out, their activities also influence your brain.
University College Cork Study PDF Download
Table of contents
- 1. The vagus nerve connects your gut and your brain
- 2. Gut bacteria talk to your brain too
- 3. Inflammation, gut bacteria, and depression
- 4. Gut bacteria make butyrate for brain health
- 5. Probiotics may help alleviate depression
- 6. Eat prebiotics to nourish probiotic bacteria
- 7. Gut microbes regulate happy hormones
- 8. Your microbiome composition and mental health
- 9. Balance is the key to happy gut bacteria
When the body is exposed to stress, it goes through a series of changes so that all energy and major resources are directed to the muscles and brain. Stress also causes the body to release cortisol, and all these factors can affect the gut microbiome.
Equally, if your gut microbiome is imbalanced (dysbiosis), then your overall mood can be affected. That’s because the activity of your gut bacteria affects stress and anxiety — a balanced microbiome can improve your stress resilience, but an imbalanced one can affect your mental health. Here’s how probiotics depression, gut bacteria and mental health are linked.
☝️TIP☝️Discover what’s living in your gut with theAtlas Microbiome Test and find out what to eat for happy bacteria.
1. The vagus nerve connects your gut and your brain
Your gut and brain are connected by the vagus nerve, a major component of the autonomic nervous system which enables you to breathe, digest food, and swallow automatically. This nerve is able to send messages to your brain for your colon, and vice versa.
The connection between the two organs means that the gut-brain axis is becoming a vital player in mental health, illnesses that affect the brain, and even irritable bowel syndrome (IBS). It explains why stress can take a toll on your digestion, but also why digestive problems can make you unhappy.
The role of the vagus nerve in digestion:
- Motility — helps food move through digestive tract
- Digestion — stimulates the release of digestive enzymes
- Appetite — communicates satiety to the brain
2. Gut bacteria talk to your brain too
On the other hand, when the vagus nerve is impaired by stress (that directs energy and attention to your muscles and brain), it can’t react effectively to inflammation, which is bad for your gut and your gut bacteria. And that’s why your vagus nerve is so important.
3. Inflammation, gut bacteria, and depression
To support your health, your gut microbiome needs to be diverse, and diversity helps keep it balanced. However, if it is not balanced — something called dysbiosis — opportunistic microbes can take advantage and proliferate, resulting in inflammation.

That’s because your body doesn’t want opportunistic bacteria, so your immune system is alerted, resulting in inflammation. Interestingly, inflammation can contribute to depression, and depression can cause inflammation. But a diverse microbiome can prevent inflammation.
So, controlling inflammation can help to improve both mood and anxiety levels. Diet is one way to increase the abundance of diverse microbes and reduce inflammation. Beneficial gut bacteria thrive on a natural, plant-based diet because fiber is an important source of energy for them.
4. Gut bacteria and mental health: the butyrate effect
butyrate is an essential short-chain fatty acid produced by good gut bacteria when you eat plants (fruit, veg, seeds, nuts, whole grains, legumes). It doesn’t just keep your gut happy, your brain benefits too. A microbiome test by AtlasBiomed can show you how much butyrate is produced by your gut bacteria.
butyrate is the main source of fuel for the cells of your gut lining, so it helps keep this barrier strong and intact. It also helps prevent inflammation, which can be bad for your mood. A new study even shows that butyrate might help you grow new brain cells. However, if you have dysbiosis, your gut bacteria might make less important nutrients, including butyrate.
What Are Short-chain Fatty Acids And Why Should You Care?
The great news is you can actively contribute to the butyrate production in your gut through your diet. One way is by eating prebiotics: foods which directly provide sustenance to your gut bacteria, like fruit, vegetables, whole grains, and pulses. These contain fibre which is transformed into SCFAs like butyrate . So, increasing your intake will positively affect your health!
5. Probiotics and depression
Probiotic bacteria provide many health benefits, including for the brain. They naturally reside in the gut but are also found in supplements and fermented foods, like yoghurt and kefir. Bifidobacterium, Lactobacillus, and Lactococcus species are all examples of probiotics because they support your whole-body and improve mental health too.

Psychobiotics is a field which investigates the effects of probiotics and mental health. Some research shows that certain Lactobacillus species improve stress resilience and anxiety. Some studies even show that taking probiotics can help alleviate symptoms of depression.
Probiotics help to support human health by keeping the gut ecosystem balanced and preventing dysbiosis. By doing so, beneficial bacteria can thrive and contribute to your health and butyrate production. The positive link between probiotics and depression is already showing great promise.
6. Eat prebiotics to nourish probiotic bacteria
So, you’ve upped your probiotic intake, but to reap all their health benefits, you need to keep them nourished. Just like you, your gut bacteria need food to keep them sustained, energised, and thriving. That’s where prebiotics come in.
Prebiotics are substances found in plant-based foods which maintain beneficial gut bacteria. Prebiotic fibres, polyphenols, and resistant starches all nourish gut bacteria which in turn transform them into good things like SCFAs and vitamins.
Prebiotic food list
| Prebiotic fibres | Resistant starches | Polyphenols |
|---|---|---|
| Garlic | Legumes | Onion |
| Onions | Seeds | Apples |
| Berries | Grains | Tea |
| Jerusalem artichokes | Cooked and cooled potatoes | Cocoa |
| Mushrooms | Green bananas | Red wine |
| Rye | Plantain | Red fruit |
| Barley | Corn | Soybeans |
Research has also shown that consuming prebiotics is also associated with a reduction in anxiety-related behaviour. So it’s important to never underestimate the role of your diet in improving mental wellbeing. You can actually get personalised food recommendations for your gut bacteria if you take a gut microbiome test like this one here.
7. Gut microbes regulate happy hormones
So, you know that your gut microbes are pretty cool and transform food into short-chain fatty acids? Well, these SCFAs communicate with cells which produce serotonin, a neurotransmitter (and a hormone) that regulates your mood, as well as levels of anxiety and happiness. Basically, your gut microbes can help your body produce more serotonin.
Equally, another neurotransmitter, Gamma-Aminobutyric Acid (GABA), regulates and improves mood because it helps to calm the nervous system and switch off stress reactions. Amazingly, some probiotic gut bacteria can even produce GABA themselves for your body.
Essential guide to happy hormones
Fundamentally, your diet can help your bacteria protect your mental wellbeing because eating the right foods feeds happy bacteria. And when you have lots of different healthy bacteria, your microbiome is more diverse and produces substances which increase mood-lifting chemicals, like serotonin and GABA.
8. Your microbiome composition and mental health
It’s clear there is a link between gut bacteria and depression. The composition of the gut microbiome can tell you a lot about what is going on inside your body. Remember that everyone’s gut microbiome is unique, but diversity is a proven factor in keeping your body (and your mind) healthy.

Fortunately, it’s now easy to get your personal microbiome health status with at-home testing. You can see how diverse your microbiome is, how well it produces butyrate , and even what foods you should eat to support a healthy and happy microbial ecosystem.
9. Balance is the key to happy gut bacteria
It’s easy to think of each of the body’s systems as separate entities, and although they may be in some respects, they are also well connected and can influence each other’s activities. The gut and the brain are prime examples of how changes in one can affect the other.
An imbalanced gut microbiome, or dysbiosis, is associated with many diseases, including mood disorders like depression. Likewise, depression can cause inflammation which can affect the natural ecosystem in the gut. However, promising research shows that probiotics and prebiotics are having positive effects on depression, anxiety, and stress resilience.
- Ansari, F et al. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders, 2020
- Benakis, C et al. The Microbiome-Gut-Brain Axis in Acute and Chronic Brain Diseases, 2020
- Cheung, S, G et al. Systematic Review of Gut Microbiota and Major Depression, 2019
- Liu, H et al. Butyrate: A Double-Edged Sword for Health? , 2018
- Liu, L and Zhu, G. Gut-Brain Axis and Mood Disorder, 2018
- Peirce, J, M and Alviña, K. The Role of Inflammation and the Gut Microbiome in Depression and Anxiety, 2018
Stakenborg, N et al. The Versatile Role of the Vagus Nerve in the Gastrointestinal Tract, 2014](https://www.researchgate.net/publication/260322955_The_Versatile_Role_Of_The_Vagus_Nerve_In_The_Gastrointestinal_Tract/link/02e7e530c79f7a692f000000/download) - Winter, G et al. Gut Microbiome and Depression: What We Know and What We Need to Know, 2018
Gut microbe linked to depression in large health study
Result brings researchers a step closer to harnessing microbes to combat mood disorders
The trillions of bacteria in and on our bodies can bolster our health and contribute to disease, but just which microbes are the key actors has been elusive. Now, a study involving thousands of people in Finland has identified a potential microbial culprit in some cases of depression.
The finding, which emerged from a study of how genetics and diet affect the microbiome, “is really solid proof that this association could have major clinical importance,” says Jack Gilbert, a microbial ecologist at the University of California, San Diego, who was not involved with the work.
Researchers are finding ever more links between brain conditions and gut microbes. People with autism and mood disorders, for example, have deficits of certain key bacteria in their guts. Whether those microbial deficits actually help cause the disorders is unclear, but the findings have spawned a rush to harness gut microbes and the substances they produce as possible treatments for a variety of brain disorders. Indeed, researchers recently reported in Frontiers in Psychiatry that fecal transplants improved symptoms in two depressed patients.
Guillaume Méric didn’t set out to find microbes that cause depression. A microbial bioinformatician at the Baker Heart & Diabetes Institute, he and his colleagues were analyzing data from a large health and lifestyle study from Finland. Part of a 40-year effort to track down underlying causes of chronic disease in Finnish people, the 2002 study assessed the genetic makeup of 6000 participants, identified their gut microbes, and compiled extensive data about their diets, lifestyles, prescription drug use, and health. Researchers tracked the health of participants until 2018.
Méric and his colleagues combed the data for clues to how a person’s diet and genetics affect the microbiome. “There have been very few studies that have examined [all these factors] in such detail,” Gilbert says. Two sections of the human genome seemed to strongly influence which microbes are present in the gut, the researchers report this week in Nature Genetics. One contains the gene for digesting the milk sugar lactose, and the other helps specify blood type. (A second study, also published today in Nature Genetics, identified the same genetic loci by analyzing the relationship between the genomes and gut microbes of 7700 people in the Netherlands.)
Méric’s team also explored which genetic variants might affect the abundance of certain microbes—and which of those variants were linked to 46 common diseases. When it came to depression, two bacteria that cause infections in hospitalized patients, Morganella and Klebsiella, seemed to play a causal role, the researchers say. One of them, Morganella, was significantly increased in a microbial survey of the 181 people in the study who later developed depression.
“This is really exciting,” says Jeroen Raes, a microbiologist at KU Leuven who was not involved with the study. “The beauty of the work,” he adds, is that Méric and colleagues made the connection between increased levels of the bacterium and patients undergoing depression.
Morganella has already been implicated in depression. As far back as 2008, researchers investigating a possible link between depression and inflammation found depressed people had stronger immune responses to chemicals produced by Morganella and other gram-negative bacteria in the gut. Thus, the newest study seems to be “further proof” that inflammation caused by gut microbes can influence mood, Gilbert says.
But the field is still in its infancy, says Gerard Clarke, a microbiome researcher at University College Cork, as there are many forms of depression and many possible ways that microbes could affect this disease. The “holy grail” is to identify a missing microbe that could be given as supplement, he says. But it’s less clear how Morganella could be eliminated from the gut to relieve symptoms. “That’s a bit more challenging.” source
Gut microbial metabolites in depression: understanding the biochemical mechanisms
Abstract
Gastrointestinal and central function are intrinsically connected by the gut microbiota, an ecosystem that has co-evolved with the host to expand its biotransformational capabilities and interact with host physiological processes by means of its metabolic products. Abnormalities in this microbiota-gut-brain axis have emerged as a key component in the pathophysiology of depression, leading to more research attempting to understand the neuroactive potential of the products of gut microbial metabolism. This review explores the potential for the gut microbiota to contribute to depression and focuses on the role that microbially-derived molecules – neurotransmitters, short-chain fatty acids, indoles, bile acids, choline metabolites, lactate and vitamins – play in the context of emotional behavior. The future of gut-brain axis research lies is moving away from association, towards the mechanisms underlying the relationship between the gut bacteria and depressive behavior. We propose that direct and indirect mechanisms exist through which gut microbial metabolites affect depressive behavior: these include (i) direct stimulation of central receptors, (ii) peripheral stimulation of neural, endocrine, and immune mediators, and (iii) epigenetic regulation of histone acetylation and DNA methylation. Elucidating these mechanisms is essential to expand our understanding of the etiology of depression, and to develop new strategies to harness the beneficial psychotropic effects of these molecules. Overall, the review highlights the potential for dietary interventions to represent such novel therapeutic strategies for major depressive disorder.
THE GUT MICROBIOME CONTRIBUTES TO DEPRESSIVE BEHAVIOR
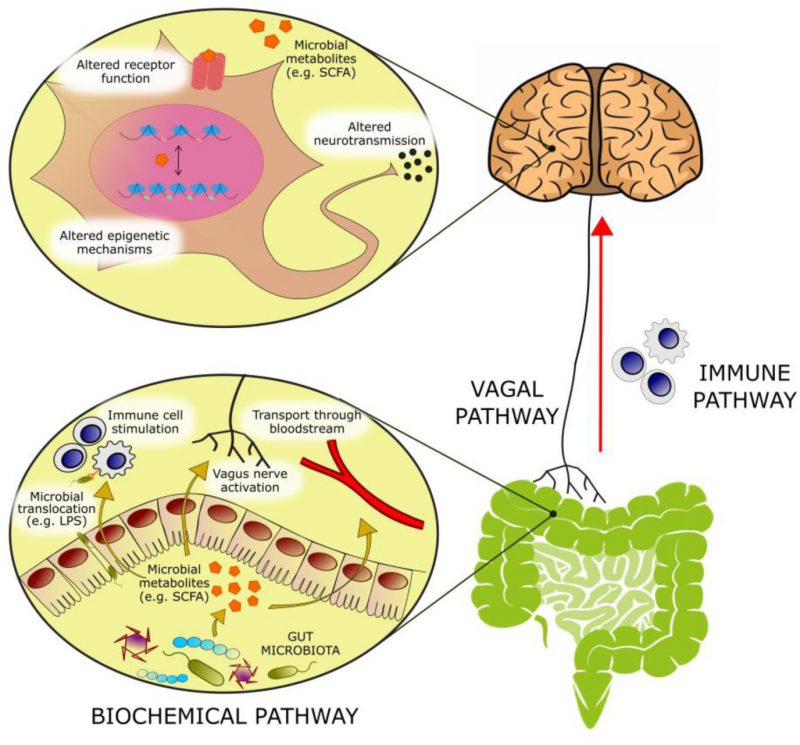
With an estimated three to four million different genes in the collective genomes of the gut microbiota [1] there is approximately 100 to 150 times more genetic information in the human microbiome than the human genome. Many of these genes encode proteins that perform metabolic functions and produce metabolites exclusive to the microbiome. The host encounters these metabolites in the gut, where they can exert local effects in the gastrointestinal (GI) environment or at the gut wall. Alternatively, these microbial metabolites can be absorbed, enter the systemic circulation and reach distant organs, including the brain. At these host sites, microbial metabolites can serve as ligands for host receptors with downstream effects on host gene expression and function. In addition, these microbial metabolites can integrate into host metabolic pathways altering their activity (Figure 1).
–
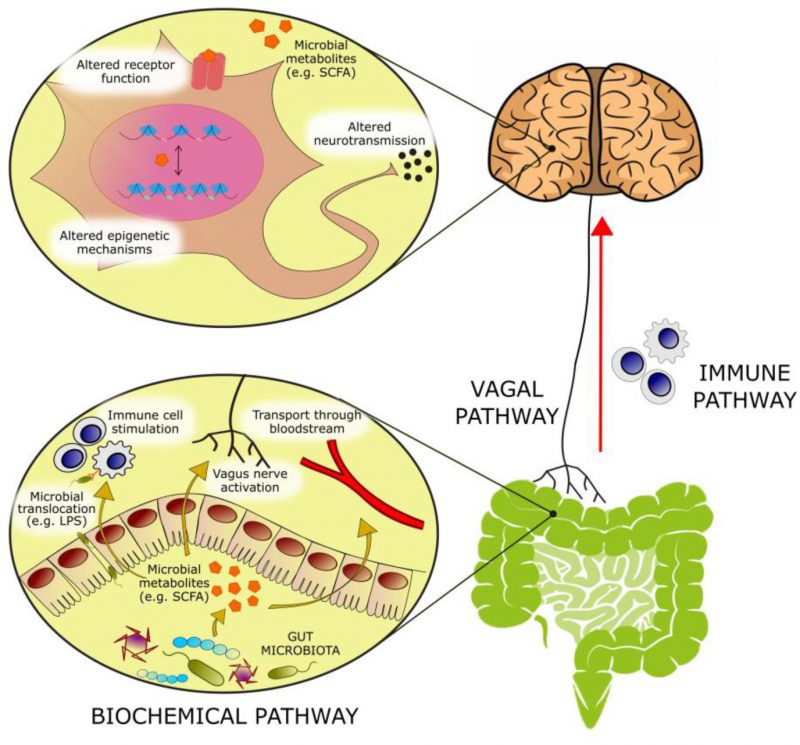 |
FIGURE 1: Bioactive molecules originating from microbial metabolism are thought to modulate emotional behavior through several mechanisms: – (1) Activation of afferent vagal nerve fibers. – (2) Stimulation of the mucosal immune system or of circulatory immune cells after translocation from the gut into the circulation. – (3) Absorption into the bloodstream, and biochemical interaction with a number of distal organs. In the brain, such metabolites may be able to activate receptors on neurons or glia, modulate neuronal excitability, and change expression patterns by means of epigenetic mechanisms. |
–
Colonization of the human gut by the microbiota is an evolutionary-driven process that impacts host physiology, for example, by priming the immune system and aiding the breakdown of otherwise indigestible fibers, and also by driving brain development and shaping behavior [2]. It is now well established that a bidirectional communication network exists between the gut and the brain, termed the gut-brain axis [3], of which the microbiota and its metabolic output are a major component. Colonization of the gut by the microbiota and central nervous system (CNS) development have extensively overlapping critical developmental windows. As a result, early-life perturbations in the maturation of the microbiota can result in deficits in neurogenesis, axonal and dendritic growth and synaptogenesis, which can negatively impact on later mental health [4]. Indeed, compared to specific pathogen-free and conventional mice, germ-free mice exhibited an exaggerated hypothalamic pituitary adrenal (HPA) axis response to restraint stress, characterized by elevated plasma adrenocorticotropic hormone (ACTH) and corticosterone as well as reduced cortical and hippocampal expression of brain-derived neurotrophic factor (BDNF) [5]. Fecal inoculation from specific pathogen-free donor mice reversed these stress-associated physiological alterations only when administered at early developmental stages. This suggests that early-life colonization by the gut microbiota is essential for the normal development of the HPA axis and of the neuroendocrine response to stress [5] and supports the notion that a limited, early critical window exists in which gut microbial stimulation shapes normal brain development [2].
–
Major depressive disorder (MDD) has become the leading cause of disability globally and is associated with death and suicide, more often than any other mental or physical disorder. The symptomatology of MDD includes prolonged feelings of low mood, worthlessness or guilt, anhedonia, sleep and appetite disturbances, fatigue, slowed movements and speech, and suicidal thoughts [6]. In addition to CNS abnormalities, patients with depression also exhibit alterations in metabolic, immune and endocrine systems. There is growing evidence associating the gut microbiota in the pathophysiology of depression. Several taxonomic association studies in humans have observed differences in the fecal microbiota composition of MDD patients compared to healthy subjects [7][8][9][10]. These studies identified variation in the phyla Bacteroidetes, Proteobacteria, Actinobacteria and Firmicutes, and in the genera Enterobacteriaceae, Alistipes, Faecalibacterium, Bifidobacterium and Blautia, although contradicting results were found regarding the direction of the associations detected between disease and bacterial taxa. Valles-Colomer and colleagues [11] used a module-based analytical approach of fecal metagenomes to link microbiota neuroactive capacity with depressive symptoms. This study showed a positive association between quality of life indicators and the genera Faecalibacterium and Coprococcus, as well as a negative association between the abundance of Coprococcus spp. and Dialister with depression after controlling for antidepressant use. Psychological stress can change the composition of the gut microbiota [12], and in turn, microbiota abnormalities can influence emotional behavior [13]. Germ-free rodent studies have begun to interrogate the causative role of microbiome abnormalities in the etiology of depression. Alongside the appearance of anhedonia and anxiety-like behavior, the oral gavage of fecal microbiota from MDD patients to antibiotic-treated rats induced decreased gut microbiota richness and diversity and elevated plasma kynurenine and kynurenine/tryptophan ratio [14], highlighting the potential to transfer depressive-like behavioral and physiological traits via the microbiota. Tryptophan metabolism along the serotonin (also known as 5-hydroxytryptamine or 5-HT), kynurenine and indole pathways can be influenced by the gut microbiota. The bacterial enzyme tryptophanase is responsible for the conversion of tryptophan into indole, which can give rise to a range of neuroactive signaling molecules. Additionally, tryptophan can be metabolized into 5-HT, via aromatic amino acid decarboxylase (AAAD) activity, or kynurenine by the enzymes tryptophan-2,3-dioxygenase (TDO) or the ubiquitous indoleamine-2,3-dioxygenase (IDO). Lipopolysaccharides (LPS), an inflammatory cell wall component from Gram negative bacteria, can induce the expression of IDO, increasing the conversion of tryptophan to kynurenine (reflected in the kynurenine:tryptophan ratio). The reduction in Firmicutes and the subsequent decrease in short-chain fatty acid synthesis observed in MDD patients has been linked to increased inflammation [15], and cytokines are also known to promote tryptophan utilization for kynurenine synthesis via IDO activity. This pathway gives rise to the neurotoxic metabolite quinolinic acid, and reduces central serotonergic availability [16]. Much of the mechanistic evidence of the involvement of the gut microbiota in depression comes from research on germ-free or on microbiota-depleted animals. Germ-free rodent models show substantial behavioral and molecular abnormalities (Table 1), represented by reduced anxiety and changes in central levels of several neurotransmitters, both of which could be rescued following colonization with a conventional microbiota early in life [17][18]. Depletion of the gut microbiota by antibiotic administration was also found to induce depressive-like behaviors in adult rats, as well as altered central 5-HT availability and other depression-related physiological changes [19].
–
|
TABLE 1. Studies investigating the effect of a lack of microbiota on neurotransmitter systems. |
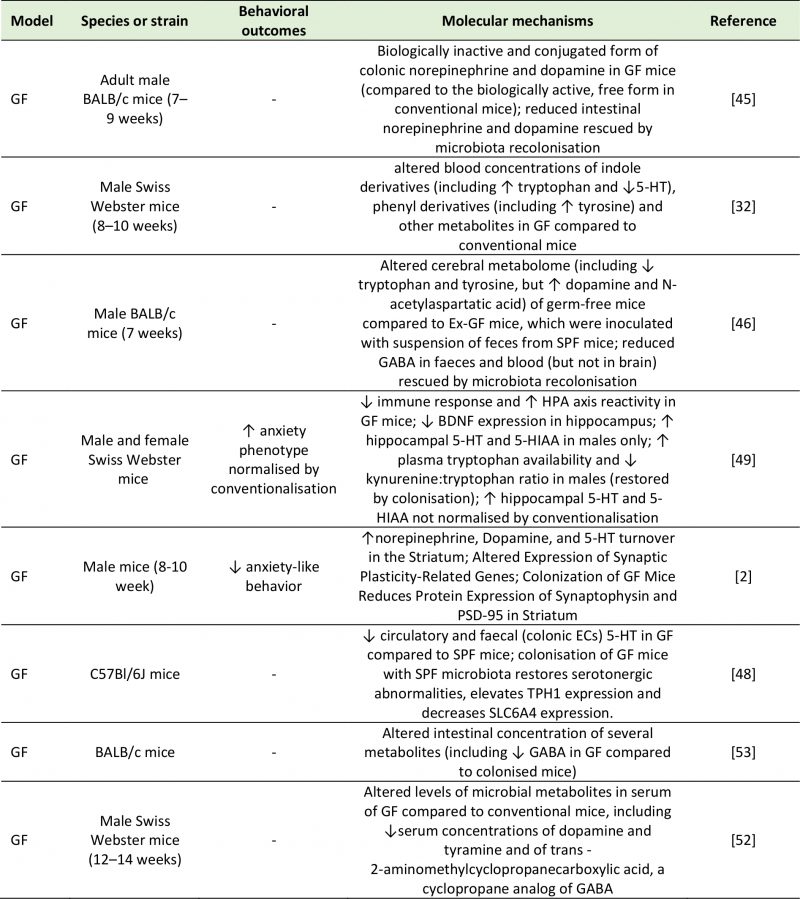 |
| 5-HIAA: 5-Hydroxyindoleacetic Acid; 5-HT: 5-Hydroxytryptamine; BDNF: Brain-Derived Neurotrophic Factor; GABA: Gamma-Aminobutyric Acid; GF: Germ-Free; HPA: Hypothalamic-Pituitary-Adrenal; PSD-95: Postsynaptic Density Protein 95; SLC6A4: Serotonin Transporter; SPF: Specific Pathogen Free; TPH-1: Tryptophan Hydroxylase 1.
[2][32][45][46][48][49][52][53] |
PATHWAYS OF MICROBIOTA-GUT-BRAIN-COMMUNICATION
The gut microbiota and its metabolic products can affect central physiological and pathological processes through several proposed mechanisms. Neural communication between the gut and the brain is mainly mediated by intestinal afferent fibers of the vagus nerve. Vagal stimulation by the gut microbiota or its metabolites is relayed to the nucleus tractus solitarius, and then transmitted to the thalamus, hypothalamus, locus coeruleus, amygdala and periaqueductal grey [3]. Electrical stimulation of the vagus nerve by the gut microbiota can alter the concentrations of neurotransmitters like 5-HT, γ-aminobutyric acid (GABA) and glutamate in the brain of both rodents and humans [20]. Additionally, rodent studies have shown that the anxiety and depressive phenotype that is normally induced by an immune challenge can be prevented by vagotomy [21][22], supporting the role played by the vagus nerve in stress reactivity and emotional regulation.
–
The immune system represents a major component of gut-to-brain communication. While central immune cells and low levels of inflammatory mediators exert a variety of physiological roles in the brain (ranging from sleep to memory formation), sustained neuroinflammation has deleterious effects on brain function and has been associated with a variety of neuropsychiatric disorders [23]. The gut microbiota has important roles in shaping immune function throughout life. In early life, it directs normal development of central immune cells, like microglia and astrocytes [24]; in adulthood, it sets a chronic physiological state of low-grade inflammation [25], as the bacterial antigens present in the intestinal tract stimulate cytokine release by intestinal macrophages and T cells [26]. Peptidoglycans derived from bacterial cell walls have been measured in the brain, where they activate central pattern-recognition receptors to stimulate the innate immune system and alter behavior [27]. These observations are consistent with a role for immune molecules in the CNS independent of infection or immune stimulation, but actually a component of normal healthy brain function.
–
The gut microbiota is also central to brain function in the context of an immune challenge. LPS can trigger the release of the cytokine IL-18 [28]. Parenteral administration of LPS to healthy individuals induced immune system activation accompanied with mild depressive and cognitive symptoms [29]. Significantly, LPS translocation into the brain is suggested to be under the control of propionate, a gut microbial metabolite that modulates blood-brain barrier (BBB) permeability [30]. Pro-inflammatory cytokines in the GI tract can also modulate central stress circuitry by stimulating the vagus nerve and activating the HPA axis [31]. Stress and dietary patterns such as the Western diet can contribute to neuroinflammation by increasing the permeability of the intestinal wall, a pathological state referred to as “leaky gut”. This allows the translocation of bacteria and LPS from the intestinal lumen into the bloodstream and the CNS [25].
–
Finally, direct biochemical signaling can take place by means of bioactive molecules of bacterial origin. Extensive studies in germ-free and antibiotic-treated rodents have highlighted the diverse biochemical output of the microbiome. This diversity is a product of the chemically heterogeneous substrate entering the gut from both the diet and host secretions as well as from the expansive metabolic repertoire of the microbiome [32]. Metabolites produced in the gut by the bacterial fermentation of dietary components can be absorbed in the bloodstream and interact with enzymes and receptors expressed by the host, contributing to both physiological and pathological processes in the host [33]. To date, evidence suggests that microbiota-derived acetate can act remotely to influence neural function [34]. Neurotransmitters, short-chain fatty acids (SCFAs), bile acids, choline metabolites, lactate and vitamins are products of gut microbial metabolism that can directly or indirectly influence central processes and, when dysregulated, contribute to neuropathology [25]. This review will focus on the potential of these metabolite classes to alter biochemical processes underlying gut-to-brain communication, and describe the role played by these microbial metabolites in the pathophysiology of depression.
NEUROACTIVE MICROBIAL METABOLITES AND THEIR ROLE IN DEPRESSION
Neurotransmitters
The majority of central neurotransmitters are also present in the GI tract, where they exert local effects ranging from modulating gut motility and secretion to cell signaling [35][36]. Members of the gut microbiota can synthesize neurotransmitters: Lactobacilli and Bifidobacteria produce GABA [37][38][39][40][41]; Escherichia coli (E. coli) produce 5-HT and dopamine [42][43]; Lactobacilli produce acetylcholine [44], and many more microbial taxa contribute to the synthesis and release of other molecules with neuroactive properties. In fact, gut microbiota absence is associated with significant reductions in intestinal levels of neurotransmitters like norepinephrine [45], 5-HT [32], and GABA [46]. While recolonization can re-establish normal neurotransmitter concentrations, it is not clear if this restoration of neurotransmission is due to bacterially derived products or due to stimulation of neurotransmitter producing host intestinal cells [47]. An example of the latter is 5-HT, whose intestinal concentrations are maintained by enterochromaffin cells, which express the enzyme tryptophan hydroxylase upon stimulation by gut metabolites such as SCFAs and secondary bile acids [48].
–
It is now established that peripheral production of neurotransmitters by the gut microbiome may alter brain chemistry and influence behavior (Table 2). While there is no evidence that gut-derived neurotransmitters reach the brain, these compounds may influence CNS signaling by co-feeding of other commensal bacteria and modulation of local host gut physiology upon absorption into the bloodstream. For example, Clarke et al. [49] showed that male germ-free mice exhibit anxiety-like behaviors as well as altered neurotransmitter (5-HT and 5-hydroxyindole acetic acid (5-HIAA)) abundance in the hippocampus. These central alterations were accompanied by an elevation in plasma tryptophan concentrations, suggesting that the peripheral tryptophan metabolism is influenced by microbiota, that also influence CNS neurotransmitter systems. While abnormal anxiety behavior was normalized by conventionalization in adulthood, the neurochemical imbalances in male germ-free mice persisted, indicating the profound effects of the gut microbiota on the development of neurotransmission [49]. The concentrations of dopamine and norepinephrine were also increased in the brains of germ-free mice in a separate study [17]. Additionally, a study chronically administering L. rhamnosus to mice reported changes in GABAA and GABAB receptor expression as well as in the levels of brain activity, accompanied by a reduction in anxiety and depression-like symptoms [50]. Similarly, the GABA-producing L. brevis FPA3709 had an antidepressant effect when administered to rats [51]. Lower circulating concentrations of 5-HT [32][48], dopamine [52] and GABA [53] have been observed in germ-free mice. This finding suggests that the gut microbiota may modulate neurotransmission via the bloodstream. Although enhancing 5-HT production in the gut does not result in an increase in central concentrations [47], central concentrations of 5-HT can be enhanced by increasing the concentrations of its precursor tryptophan in the GI tract [16][54]. These findings have an important relevance in the context of depression, as they demonstrate the possibility of modulating central serotonergic neurotransmission through non-invasive interventions that target the gut microbiome.
–
|
TABLE 2. Studies investigating the effects of treatment with microbial cultures on neurotransmission and behavior. |
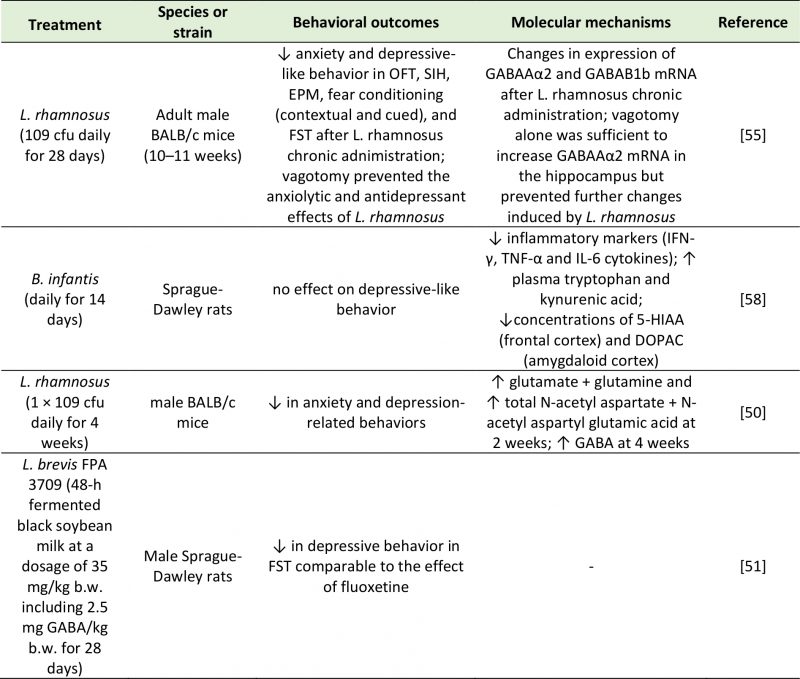 |
| 5-HIAA: 5-Hydroxyindoleacetic Acid; cfu: Colony-Forming Unit; DOPAC: 3,4-Dihydroxyphenylacetic Acid; FST: Forced Swim Test; EPM: Elevated Plus Maze; GABA: Gamma-Aminobutyric Acid; IFN-γ: Interferon Gamma; IL-6: Interleukin-6; OFT; Open Field Test; SIH: Stress-Induced Hyperthermia; TNF-α: Tumor Necrosis Factor Alpha.
[50][51][55][58] |
–
Microbial metabolites can also have an impact on central neurotransmission by activating afferent nerve fibers. The involvement of the vagus nerve in gut-brain communication was demonstrated by Bravo et al. [55]. This work showed that administration of probiotics like L. rhamnosus had anxiolytic and antidepressant effects and induced significant changes in GABA receptor expression in the brain of normal, but not vagotomized, mice [55]. Neurotransmitters produced in the gut may also influence brain function through the modulation of the immune system. There have been reports of 5-HT activating cells of the immune system [56], and of GABA dampening intestinal inflammation [57]. Upon chronic administration of the probiotic B. infantis, naïve rats displayed an attenuation of inflammatory markers such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [58]. Since a concomitant increase in circulating tryptophan and kynurenic acid and decrease in central 5-HIAA and 3,4-dihydroxyphenylacetic acid (DOPAC) were described, the dampening of the inflammatory response may be ascribed to a change in neurotransmitter production and availability [58]. Alternatively, neurotransmitters produced by the gut microbiota can inhibit cytokine production through local stimulation of the vagus nerve [59].
–
These studies suggest that neurotransmitters produced, either directly or indirectly, by gut bacteria may influence emotional behavior by binding specific receptors in the CNS, or peripheral receptors on neural or immune cells. A wider range of bacterially-derived, bioactive, transmitter-like molecules may exist whose effects on depressive symptoms have not been investigated to the same extent as classic neurotransmitters. These molecules include histamine, gasotransmitters (e.g. nitric oxide, ammonia), neuropeptides, endocannabinoids, steroids [60], and it is likely that more will be identified in the future. This communication between bacterial and host metabolism of neurotransmitters is bidirectional in nature: in addition to synthesizing neurotransmitters that are able to alter host physiology, gut microbes can also respond to neurotransmitters produced by the host, which influence bacterial growth and development [61].
–
SCFAs
SCFAs are small organic compounds produced in the cecum and colon by anaerobic fermentation of predominantly indigestible dietary carbohydrates that cross-feed other bacteria and are readily absorbed in the large bowel [62]. SCFAs are involved in digestive, immune and central function, although different accounts on their impact on behavior exist. Administration of the three most abundant SCFAs (acetate, butyrate and propionate) was shown to alleviate symptoms of depression in mice [63]. In support of their involvement with the etiology of depression, a depletion of butyrate , acetate and propionate was reported in MDD patients [8][10][64][65], and a high abundance of butyrate -producing bacteria, like Faecalibacterium and Coprococcus spp., was detected in subjects with higher quality of life indicators [11]. The genera Faecalibacterium and Coprococcus are Gram-positive, anaerobic bacteria which ferment dietary fibers to produce SCFAs. Faecalibacteria are one of the most abundant gut microbial genera, with important immunological functions and clinical relevance for a variety of diseases, including MDD [8].
–
SCFAs are able to bind and activate the G protein-coupled receptors GPR43 (free fatty acid receptor 2 (FFAR2)) and GPR41 (FFAR3), as well as the less common CPR164 and GPR109a (also known as OR51E1 and HCAR2 respectively) [66]. These receptors are ubiquitously expressed by several organs in the body, including enteroendocrine cells, adipocytes, immune cells and neurons [66], suggesting that SCFAs may alter behavior by direct stimulation of neural pathways, or through the indirect central effect of neuroendocrine and immune activation.
–
Locally, SCFAs promote gut health by modulating energy regulation, glucose metabolism and lipid homeostasis [67] and regulate intestinal barrier integrity by enhancing the expression of tight junctions (particularly butyrate , see [68]). By binding to FFAR2, SCFAs control feeding behavior by stimulating the production of the anorexigenic hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) by enteroendocrine cells [69][70][71], and of leptin by adipocytes [72]. As previously stated, SCFAs also contribute to the synthesis and release of peripheral neurotransmitters (like 5-HT and acetylcholine) by enterochromaffin cells, in a process that is thought to be mediated by OR51E1 [73], and norepinephrine by sympathetic neurons, via stimulation of FFAR2 and FFAR3 [74]. Recent work has demonstrated the presence of FFAR3 in the mouse vagal ganglia [75], suggesting a role for SCFAs in establishing visceral reflexes. The ability of SCFAs to activate vagal fibers and induce activity in the hypothalamus has been implicated as the neural basis of their central anorexigenic effect [76]. In addition to their local action in the gut and in the peripheral nervous system, SCFAs can act directly on central receptors due to their ability to diffuse passively or actively (via monocarboxylate transporters) across the BBB [77][78]. SCFAs like acetate can directly modulate appetite by binding to and activating receptors in the hypothalamus [34]. Interestingly, appetite suppression by propionate involves the attenuation of neural activity in regions of the brain reward system (i.e. caudate and nucleus accumbens) [79], a circuitry that is also dysfunctional in patients with depression [80]. Since no change in circulatory concentrations of PYY or GLP-1 were observed, it is likely that signaling via the vagus nerve or central receptors is responsible for the central effects of propionate. In addition, in vitro studies show both propionate and butyrate , but not acetate, can modulate the permeability of the BBB, protecting against the increased permeability caused by LPS [30].
–
Binding of SCFAs to FFAR2, FFAR3, GPR109a and Olfr78 receptors expressed by immune cells contributes to the development and function of the immune system [81]. For example, microglia abnormalities in germ-free mice can be reversed by SCFA administration in a FFAR2-dependent manner [82]. The observation that SCFAs generally dampen inflammation [83][84] suggests that the antidepressant effects of SCFAs may be partly accounted for by their anti-inflammatory properties. However, while butyrate was shown to suppress neuroinflammation by acting on microglial GPR109a receptors [68], propionic acid was shown to activate microglia and induce reactive astrogliosis in rats [85] and to promote immune cell recruitment in a FFAR3-mediated pathway [86]. These observations suggest a complex relationship between SCFAs and immune function. Moreover, while central butyrate promotes neurogenesis and angiogenesis [87][88] and contributes to tight junction expression and BBB structural integrity [68], intraventricular infusions of propionate contributed to mitochondrial dysfunction and oxidative stress by inducing lipid peroxidation, protein carbonylation and metabolic alterations in the rat brain [85][89][90]. However, it must be noted that many of these preclinical studies used supraphysiological doses of propionate and that, while intraventricular injections elicited a strong effect on the brain, such changes do not occur if SCFAs were administered peripherally.
–
SCFAs are strong epigenetic modulators that can control the accessibility of genetic material for DNA methylation and inhibition of histone deacetylation. A rodent study revealed the DNA methylation properties of sodium butyrate, the salt form of butyric acid [91]. This mechanism is dependent on ten-eleven translocation (TET) proteins, which catalyze the hydroxylation of cytosine residue (5mC) into 5-hydroxymethylcytosine (5hmC). 5hmC can then mediate active DNA demethylation. While depressed mice exhibited low levels of the TET methylcytosine dioxygenase 1 (TET1), mice treated with sodium butyrate showed a normalization in 5-hydroxymethylation levels by TET1, resulting in BDNF gene overexpression [91]. Depression is often characterized by altered histone deacetylase (HDAC) activity, and several studies have demonstrated the epigenetic potential of different antidepressant medications [92]. Butyrate has been identified as a HDAC inhibitor for HDAC1, HDAC2 and HDAC7 [93], and its systemic administration induced histone acetylation in the hippocampus and frontal cortex in mice [94]. The beneficial effect of sodium butyrate on mood was shown in rodent models of depression either alone [95][96][97] or in conjunction with antidepressant drugs [94][98]. For example, repeated injections of sodium butyrate reversed the LPS-induced activation of microglia and depressed mood in mice [99]. This antidepressant effect was mediated by the acetylation of hippocampal histones H3 and H4, which reduced the expression of Iba1, a marker of microglia activation [99]. Alternatively, Sun et al. [100] found that the beneficial effects of sodium butyrate on depressive behavior were mediated by an increase in 5-HT concentrations, reversal of hippocampal neuronal abnormalities, increased BDNF expression, and an upregulation of tight junction expression at the BBB [100]. In line with these findings, sodium butyrate was reported to promote the expression of dopamine, adrenaline, and other neurotransmitter genes in a rat pheochromocytoma cell line [101]. Other investigations demonstrated that further effects of HDAC inhibition by butyrate included a reduction in neuroinflammation through modulation of microglia activation [102] and an enhancement in N-methyl-D-aspartate (NMDA) receptor activity [103].
–
The SCFA propionate also acts as a HDAC inhibitor [104], and intrarectal administration of sodium propionate was shown to improve despair behavior in rats [105]. The antidepressant effect of propionate was accompanied by an increase in norepinephrine, dopamine, tryptophan, 5-HIAA and 3-hydroxyanthranilic acid (3-HAA) in the prefrontal cortex, although no change was detected in 5-HT and 3-hydroxykynurenine (3-HK). The known ability of propionate (shared with butyrate ) to promote dopamine and norepinephrine synthesis by enhancing the transcription of the tyrosine hydroxylase gene [101], may be the mechanism underlying these molecular and behavioral effects. Both butyrate and propionate may also contribute to dopaminergic function by inhibiting the expression of dopamine-β-hydroxylase, which catalyzes the conversion of dopamine into norepinephrine. Thus, the opposite effects of SCFAs on behavior may be explained by their action via independent mechanisms: for example, Li et al. [105] found that while butyrate modulated the expression of 5-HT (with slow-onset but long-term antidepressant action), propionate altered the expression of norepinephrine (with fast-acting, but short-term antidepressant action). However, propionate is also able to modulate serotonergic function by increasing the expression of tryptophan hydroxylase (TPH) [101], responsible for the conversion of tryptophan to 5-HT. This finding is significant for unveiling the link between neuroinflammation and neurotransmitter production, as an increased TPH turnover induces an accumulation of kynurenine and neurotoxic metabolites like 3-HK [105]. Since altered tryptophan–kynurenine metabolism is characteristic of depression [106], this observation suggests that serotonergic function may be linked to anti-inflammatory mechanisms. Indeed, oral administration of propionate was shown to result in a decrease in the neurotransmitters GABA, 5-HT, and dopamine, as well as in a range of biomolecular alterations which included increased oxidative stress (indicated by lipid peroxidation), altered energy metabolism, and higher pro-inflammatory markers like IL-6, TNF-α, IFN-γ, heat shock protein 70 and caspase 3 [107]. Several additional studies suggested that modulation of mood by SCFAs can occur via mechanisms involving the immune system, but the findings are contradictory. While butyrate has established anti-inflammatory effects including the inhibition of pro-inflammatory gene expression [108][109][110], propionate has been reported to have both anti- [111] and pro-inflammatory properties [112][113][114].
–
Despite this evidence (Table 3), results supporting the antidepressant potential of SCFAs are not consistent enough to be translated into medical practice. For example, cecal isobutyrate is reduced in response to administration of probiotics with antidepressant efficacy [115], and some studies have failed to detect significant abnormalities in the abundance of butyrate in MDD patients [116][117] or animal models of depression [105] compared to controls. Such discrepancies may be partly due to the highly volatile nature of SCFA and to their sensitivity to the conditions of storage and tissue extraction [118], which can affect quantification and hinder comparable results across studies. In addition, controversies exist regarding the appropriate control for studies that administer SCFA in the form of salt. Although the ideal control for this experimental model should be sodium matched, some behavioral and/or physiological effects cannot be excluded [63], especially in the light of recent findings showing that a diet high in salt alters gut microbiota composition and reduces butyrate production [119]. As for propionate, its dysregulation in animal models of depression has been consistently demonstrated [64][105], but its neurotoxic effects and the behavioral deficits elicited at excessive doses imply that more in-depth knowledge of the underlying mechanisms are required before a targeted intervention can be developed.
–
|
TABLE 3. Studies investigating the effects of SCFAs on depressive-like behavior. |
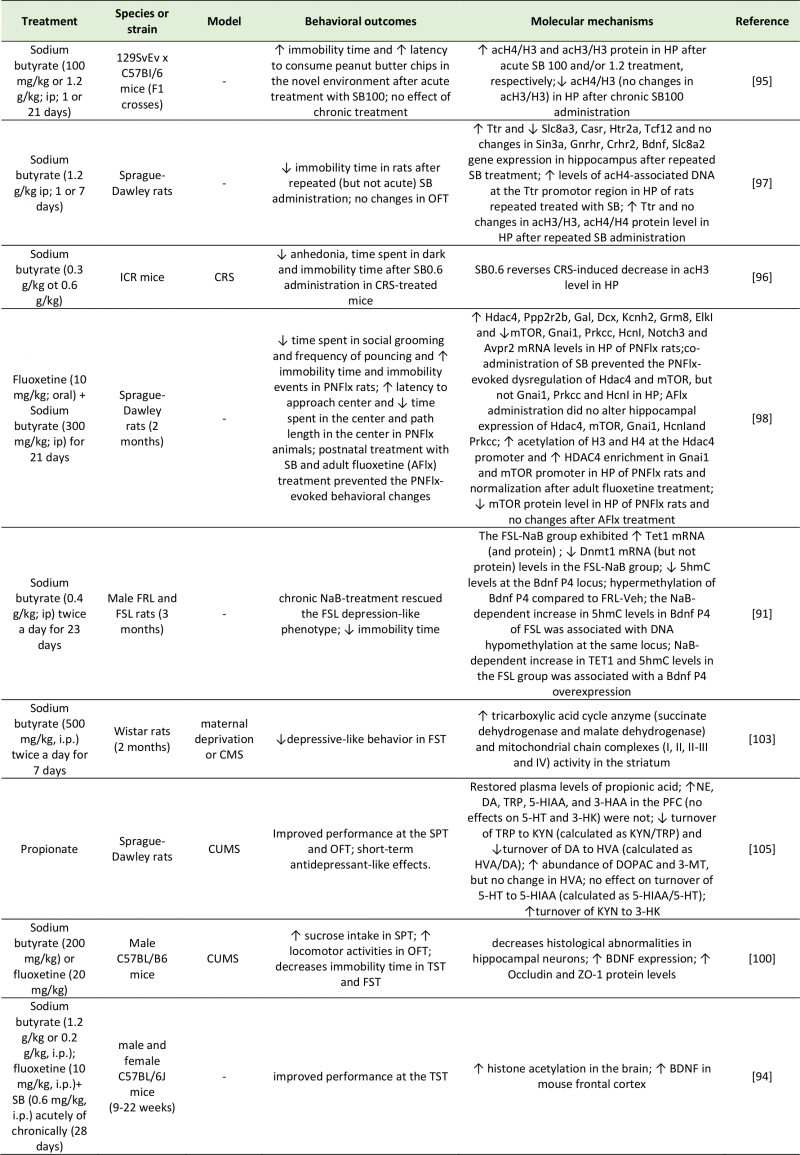 |
| 3-HAA: 3-Hydroxyanthranilic Acid; 3-HK: 3-Hydroxyanthranilic Acid; 3-MT: 3-Methoxytyramine; 5-HIAA: 5-Hydroxyindoleacetic Acid; 5hmc: 5-Hydroxymethylcytosine; 5-HT: 5-Hydroxytryptamine; Ach4/H3: Acetylated Histone H3/4; Avpr2: Arginine Vasopressin Receptor 2; Casr: Calcium-Sensing Receptor; CMS: Chronic Mild Stress; Crhr2: Corticotropin Releasing Hormone Receptor 2; CRS: Chronic Restraint Stress; CUMS: Chronic Unpredictable Mild Stress; DA: Dopamine; Dcx: Dublecortin; Dnmt1: DNA (Cytosine-5)-Methyltransferase 1; DO-PAC: 3,4-Dihydroxyphenylacetic Acid; Elkl: ETS Domain-Containing Protein; FRL: Flinders Sensitive Line; FSL: Flinders Resistant Line; FST: Forced Swim Test; Gal: Galanin; Gnai1: G Protein Subunit Alpha I1; Gnrhr: Gonadotropin Releasing Hormone Receptor; Grm8: Glutamate Metabotropic Receptor 8; Hcnl: Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel 1; Hdac4: Histone Deacetylase 4; Htr2a: 5-Hydroxytryptamine Receptor 2A; HVA: Homovanillic Acid; Kcnh2: Potassium Voltage-Gated Channel Subfamily H Member 2; KYN: Kynurenine; Mtor: Mammalian Target of Rapamycin; NE: Norepinephrine; Notch3: Neurogenic Locus Notch Homolog Protein 3; OFT: Open Field Test; PFC: Prefrontal Cortex; Ppp2r2b: Protein Phosphatase 2 Regulatory Subunit Beta; Prkcc: Protein Kinase C Gamma; Sin3a: SIN3 Transcription Regulator Family Member A; Slc8a3: Solute Carrier Family 8 Member A3; SPT: Sucrose Preference Test; Tcf12: Transcription Factor 7-Like 2; TET1: Ten-Eleven Translocation 1; TRP: Tryptophan; TST: Tail Suspention Test; Ttr: Transthyretin; ZO-1: Zonula Occludens-1.
[91][94][95][96][97][98][100][103][105] |
–
For example, there is still a lack of consensus regarding the mode of action and receptor specificity of SCFAs. In addition, it remains unclear how well the microbial production of SCFAs in the gut parallels CNS availability. It is known that lumen concentrations of SCFAs are highly variable among individuals, and can range between 20-140 mM depending (among other factors) on fiber content of the diet, microbiota composition, rate of absorption and site of measurement in the gut [120][121]. Absorbed by colonocytes, SCFAs are transported to the liver and then enter the systemic circulation in much lower concentrations (0.1–10 mM) [122][123]. Although it remains unclear how well the microbial production of SCFAs in the gut relates to CNS availability, rodent studies have shown that ~3% of acetate administered intravenously reaches the CNS [34], suggesting that only a small proportion of the SCFAs absorbed from the gut reaches the brain. Increasing bacterial production of SCFAs by means of higher fiber intake (reviewed in [124][125]) and pre- or probiotics use [126][127] have been shown to effectively enhance the concentrations of SCFAs in the gut. The question remains as to whether direct SCFA supplementation is more effective than strategies targeting the gut microbiota. While direct supplementation with SCFAs may overcome problems related to competition of probiotic strains with resident bacterial strains, care has to be taken to elucidate the effects of SCFA depending on whether it is administered acutely (i.e. via supplementation) or chronically (i.e. via microbial production). Thus, the best strategy to implement the known beneficial effects of SCFAs on mood has still to be elucidated.
–
Tryptophan metabolites
Tryptophan is an essential amino acid involved in protein synthesis [128]. Its metabolic breakdown by host (TDO and IDO) and bacterial enzymes (tryptophanase) give rise to neuroactive molecules with established mood-modulating properties, including 5-HT, kynurenine and indole. It is well-established that dietary intake of tryptophan can modulate central concentrations of 5-HT in humans [129][130], and that tryptophan depletion exacerbates depressive symptoms in healthy individual at risk for depression [131][132], as well as remitted [133][134][135] and currently depressed patients [136][137]. However, less than 5% of tryptophan is converted into 5-HT along the methoxyindoles pathway by the enzyme tryptophan hydroxylase; the remaining 95% is metabolized along the kynurenine pathway by the enzymes TDO and IDO. Kynurenine can be further metabolized into kynurenic acid (KYNA) or, alternatively, into quinolinic and picolinic acids via the nicotinamide adenine dinucleotide (NAD) pathway. KYNA is an NMDA and α7 nicotinic acetylcholine receptor antagonist; quinolinic and picolinic acids are NMDA agonists with neurotoxic and pro-depressant effects [138]. Over-stimulation of the kynurenine pathway leads to increased lipid peroxidation and inflammation, due to quinolinic and picolinic acids and free radical generation (3-hydroxykynurenine and 3-hydroxyanthranilic acid) [139][140]. Conversely, production of stress hormones (i.e. cortisol) and pro-inflammatory cytokines (i.e. interferons, TNF-α, interleukins) stimulate TDO and IDO formation respectively, enhancing kynurenine output at the expenses of 5-HT synthesis. In turn, the weakening of the inhibitory feedback of 5-HT on cortisol production contributes to the worsening of this cycle [141]. Therefore, disturbances in tryptophan metabolism (i.e. the shunt of tryptophan from 5-HT to kynurenine synthesis) may be partly responsible for the mood, cognitive and sleep disturbances typical of depression [141].
–
The mechanisms that control the uptake of tryptophan into the brain are not fully understood: these include the proportion of circulatory tryptophan that is bound to albumin (which is unable to cross the BBB), as well as the competition with other neutral amino acids for its transport through the BBB [142], but other factors are likely to be involved. Studies on germ-free animals have demonstrated the role of the microbiome in mediating the behavioral effects of tryptophan metabolism, suggesting a potential additional mechanism. Upon colonization of these animals with tryptophan-metabolizing bacteria, a decrease in tryptophan and an increase in hippocampal 5-HT concentrations was noted, accompanied by reduced anxiety-like behaviors [2][49]. Studies have shown that the metabolic activity of the gut microbiota on dietary tryptophan produces biologically active signaling molecules, such as indole and its derivatives. Indole is an aromatic amino acid produced through the microbial metabolism of tryptophan by bacteria expressing the enzyme tryptophanase (e.g. E. coli [143] and other strains [144]). In microbial communities, indole is used as a quorum-sensing signal to coordinate collective behaviors like spore formation, plasmid stability and drug resistance [144]. Moreover, it plays an important role in gut physiology as it stimulates enteroendocrine L cells to secrete GLP-1 [145] and regulates gut barrier permeability [146]. In addition, oxindole and isatin (2,3-dioxoindole), products of indole oxidation and conjugation respectively, have been described as neuroactive signaling molecules able to modulate motor function and emotional behavior. Oxindole is a strong inhibitor of motor activity, and it is known to result in loss of the righting reflex, hypotension, and reversible coma [147]. Isatin increases water intake and decreases food intake. A rodent study using antagonists selective to specific receptors highlighted the possibility of these effects being mediated by the 5-HT3 receptor and the dopamine D2 receptor [148]. The action of isatin on 5-HT3 and atrial natriuretic peptide (ANP) receptors may also be responsible for the negative effect of this compound on memory formation [149]. Additionally, isatin is an endogenous monoamine oxidase (MAO) B inhibitor and a benzodiazepine receptor antagonist. As such, it has an established anxiogenic profile in both mice and rats [150][151], and in turn, its production is drastically increased in conditions of stress. However, it is important to state that modifications in the chemical structure of indole and derivatives have been reported to drastically change the behavioral properties of these compounds, and even confer some antidepressant actions [152].
–
Based on research studies investigating the behavioral effects of indole and its metabolites, several pathways may mediate the neuroactive potential of indoles (Table 4). Enhanced tryptophan catabolism into indoles may mimic the reversible effect of a tryptophan-deficient diet, which is also associated with reduced 5-HT availability and increased neuroinflammation [153]. Other mechanisms may include direct effects of indole metabolites on central receptors, activation of the vagus nerve by gut bacteria or their metabolites, and stimulation of a neuroinflammatory state. A study by Jaglin et al. [154] showed how the effects of indole on physiology and behavior were mediated by different pathways depending on whether they were administered chronically or acutely. Acute administration of indole in the rat cecum caused a significant reduction in locomotion and an accumulation of indole metabolites in the brain, suggesting a possible direct role on central receptors. In contrast, chronic exposure to indole, achieved by the colonization of germ-free rats with E. coli, exacerbated anxiety-like and helplessness (i.e. depression-like) behaviors, but had no effect on motor activity [154]. In contrast to acutely administered animals, these colonized rats did not exhibit increased oxindole and isatin in the brain, nor increased circulatory corticosterone. These findings suggest that the behavioral alterations induced by chronic indole production (via colonization with indole-producing E. coli) are not mediated by the action of indole or its metabolites on central receptors or on the HPA axis [154]. A reduction in eye blinking frequency was detected, suggesting the involvement of the vagus nerve in eliciting the anxiogenic and depressive-like behaviors described [154].
–
|
TABLE 4. Studies investigating the effects of indole metabolites on depressive-like behavior. |
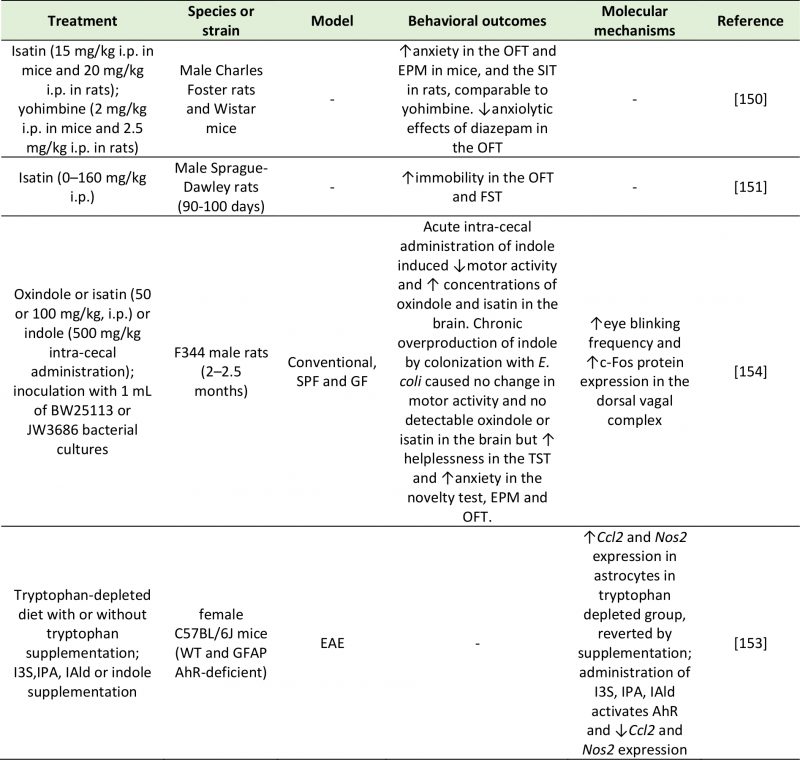 |
| AhR: Aryl Hydrocarbon Receptor; Ccl2: C-C Motif Chemokine Ligand 2; EAE: Experimental Autoimmune Encephalomyelitis; EPM: Elevat-ed Plus Maze; FST: Forced Swim Test; GF: Germ Free; GFAP: Glial Fibrillary Acidic Protein; I3S: Indoxyl-3-sulfate; IAld: Indole-3-aldehyde; IPA: Indole-3-propionic acid; Nos2: Nitric Oxide Synthase 2; OFT: Open Field Test; SIT: Social Interaction Test; SPF: Specific Pathogen Free; WT: Wild Type.
[150][151][153][154] |
–
Indole and its derivatives (e.g. indoxyl-3-sulfate (I3S), indole-3-propionic acid (IPA) and indole-3-aldehyde (IAld)) are able to activate the aryl hydrocarbon receptor (AhR) [153][155], with a subsequent inhibitory effect on neuroinflammation. Rothhammer et al. [153] showed in mice that were either supplemented with indole and related compounds or treated with tryptophanase, that neuroinflammation was reduced via activation of the AhR on astrocytes. This was attributed to increased expression of suppressor of cytokine signaling 2 (Socs2), and a subsequent inhibition of the transcription factor NF-kB.
–
Our understanding of the physiological and pathological role of indoles is hindered by the existence of a high number of indole derivatives, with diverse and dynamic actions. For example, IAld triggers the release of the anti-inflammatory cytokine IL-22 [156], IPA regulates intestinal barrier function via pregnane X receptor (PXR) [157] and is protective against DNA damage, lipid peroxidation and amyloid-β deposition in the brain [158][159], and I3S is cytotoxic and triggers free radical production [160]. Additionally, there is a very small number of studies aimed at investigating the effect of these bioactive compounds on behavior. Given the tight link between tryptophan metabolism and mood, it is important to investigate the role of these molecules in order to understand the underlying mechanisms of this disease.
–
Lactate
Lactate is an organic acid arising from both mammalian host processes and the fermentation of dietary fibers by lactic acid bacteria (e.g., L. lactis, L. gasseri, and L. reuteri), Bifidobacteria and Proteobacteria [161]. Lactate can be converted by several bacterial species to SCFAs contributing to the overall pool. Although present in the gut at low levels, lactate is absorbed into the bloodstream [162] and can cross the BBB [163]. Lactate has an established role in central signaling: in the brain, it is used as an energy substrate by neurons (due to its ability to be metabolized into glutamate) [164], it contributes to synaptic plasticity, and underlies memory formation [165][166]. Both rodent and human studies support an association between depression and lactate abnormalities (Table 5). Increased concentrations of urinary lactate were measured in patients suffering from severe MDD compared to controls [167]. Interestingly, compared to conventionally colonized mice, germ-free mice exhibit elevated hippocampal concentrations of lactate, but decreased concentrations in the frontal cortex. In contrast, germ-free rats exhibit higher frontal concentrations of lactate than conventional rats [168].
–
|
TABLE 5. Studies investigating the effects of lactate on depressive-like behavior. |
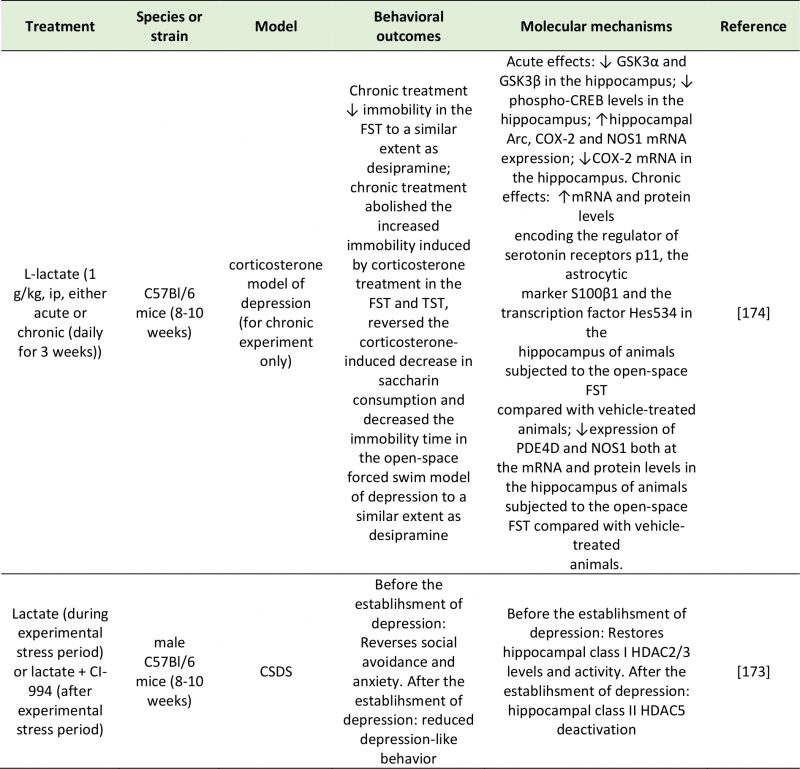 |
| Arc: Activity-Regulated Cytoskeleton-Associated Protein; COX-2: Cyclooxygenase 2; CREB: Camp Response Element-Binding Protein; CSDS: Chronic Social Defeat Stress; FST: Forced-Swim Test; GSK3α/β: Glycogen synthase kinase 3 alpha/beta; HDAC2/3/5: Histone Deacetylase 2/3/5; NOS1: Nitric Oxide Synthase 1; PDE4D: Camp-Specific 3′,5′-Cyclic Phosphodiesterase 4D; TST: Tail Suspention Test.
[173][174] |
–
A potential mechanism through which lactate can modulate emotional behavior is through direct activation of the receptor GPR81 (also known as hydroxycarboxylic acid receptor 1 or HCA1), expressed in the hippocampus, neocortex and cerebellum [169]. The involvement of GPR81 in mood disorders has been suggested by Shoblock et al. [170]. However, through GPR81 activation, lactate modulates lipid and glucose metabolism, exerts an anti-inflammatory effect (also mediated by ARRB2) [171], and inhibits GABAergic neurotransmission [172].
–
An alternative, and significantly more explored, mechanism explaining the effect of lactate on depressive behavior is epigenetic regulation of depression-related genes. An interesting study by Karnib et al. (2019) revealed that lactate has both protective and reversing effects against depression, and that these processes occur via distinct epigenetic mechanisms on HDACs [173]. In this experiment, chronic lactate administration immediately before a 10-day social defeat challenge protected against the resulting social avoidance and anxiety behaviors observed in control mice. Lactate-treated mice exhibited increased levels and activity of the class I HDAC2/3 in the hippocampus [173]. In a second group of mice, which were not given lactate during the social stress challenge period, and that exhibited depressive-like symptoms, lactate had an antidepressant effect as shown by the rescue of social avoidance behavior. After the establishment of depression, the effect of lactate was not mediated by HDAC2/3; instead, it was mediated by a reduction in HDAC5 levels [173].
–
Carrard et al. (2018) also demonstrated the antidepressant effect of acute and chronic intraperitoneal injections of L-lactate in a corticosterone mouse model of depression. These behavioral effects followed an increase in the hippocampal concentrations of L-lactate, and were dependent on changes in the expression of several genes implicated in the pathophysiology of depression: GSK-α, GSK-β and CREB phosphorylation levels were significantly decreased, while the expression of Arc was increased and COX-2 and NOS1 decreased [174]. In addition to changes in the expression of depression-related or plasticity-related genes (GSK-α, GSK-β, CREB, Arc, COX-2 and NOS1), the behavioral effects of lactate were mediated by an increase in hippocampal p11 (regulator of 5-HT receptors), S100 β (astrocytic marker), Hes5 (transcription vector) and a decrease in cAMP-specific phosphodiesterase-4D (PDE4D) and NOS1 mRNA and protein levels [174].
–
Since lactate can also be synthetized by astrocytes on neuronal demand as a byproduct of glycolysis [175], it remains difficult to assess the net effect of microbial metabolism on central levels of lactate and mood. A simple way to isolate the contribution of the gut microbiome in the relationship between lactate production and depressive behavior would be using germ-free rodents; to the best of our knowledge, this has not been investigated to date. However, the well-established interchange of lactate between the periphery and the CNS [163] points towards a role of the gut microbiota in mediating the antidepressant effects of lactate. In support of this statement, the beneficial effects of exercise on mood have been hypothesized to be due to gut microbiota-mediated changes in the production of lactate [176][177].
–
Bile acids
Bile acids are cholesterol-derived steroid acids synthesized in the liver, secreted into the small intestine and absorbed in the ileum. The two primary bile acids (in humans and rats), cholic acid (CA) and chenodeoxycholic acid (CDCA), undergo further structural modifications in the gut by means of the gut microbiota, which convert them into secondary and tertiary bile acids [178]. Bile acids have local detergent properties that enables them to emulsify lipophilic molecules and, in turn, facilitate nutrient digestion and absorption. However, they can also act as signaling molecules to modulate feeding behavior and in turn, control glucose homeostasis, lipid metabolism and energy expenditure [179]. Their signaling pathways are initiated by their binding to the farnesoid X receptor (FXR) and the Takeda G protein-coupled receptor 5 (TGR5) [180].
–
The FXR is a nuclear receptor that is involved in the synthesis, secretion and transport of bile acids [181], as well as in the modulation of CREB activity [182]. Through its inhibitory control of the transcription factor CREB, bile acids can repress the transcription of several genes, including BDNF. Since the first reports of FXR expression in the brain [180][183], the possibility has been explored that BDNF abnormalities found in the brains of depressed individuals may be accounted for, in part, by altered bile acid activity. Supportive of this hypothesis, the chronic unpredictable mild stress (CUMS) rodent model of depression exhibits enhanced hippocampal FXR expression, and in turn, FXR overexpression in the rat hippocampus is sufficient to induce depressive-like behavior in naïve animals [184]. These behavioral changes were mirrored by a significant decrease in BDNF expression in the hippocampus of rats overexpressing FXR. In contrast, FXR knockdown in naïve rats had a strong antidepressant effect as measured by the forced-swim and tail suspension tests, and prevented the occurrence of CUMS-associated behavioral (depressive-like symptoms) and molecular (decreased BDNF expression) abnormalities [184]. The antidepressant effect of FXR genetic deletion was confirmed in an independent study, which also reported altered glutamatergic, GABAergic, serotonergic, and noradrenergic neurotransmission in the hippocampus and cerebellum of FXR knockout mice, while no change was detected in the prefrontal cortex [185]. Deletion of FXR also led to disrupted bile acid metabolism and to increased bile acid abundance both peripherally and centrally [185][186]. Different rodent models of depression have reported increased abundance of bile acids in urine and plasma [187], as well as in the fecal metabolic phenotype [188]. Su et al. [189], instead, reported an upregulation in serum glycocholic acid, but a decrease in cholic acid in chronic variable stress (CVS)-induced depression rats. These abnormalities were associated with a reduced abundance of Peptostreptococcaceae incertaesedis [188], supporting a link with altered microbiota function.
–
Moreover, bile acids may contribute to major depression by disrupting tight junction expression, leading to permeabilization of both intestinal and central epithelial cells [190]. Chenodeoxycholic acid or deoxycholic acid injections permealized the BBB in naïve rats [190]. When investigated in rat brain microvascular endothelial cells, increased BBB permeability upon administration of chenodeoxycholic acid or deoxycholic acid was found to be mediated by occludin phosphorylation in a Rac-1-dependent and FXR-independent fashion [190]. Enhanced permeabilization of intestinal epithelial barrier in human Caco-2 monolayers was associated with phosphorylation of the epithelial growth factor (EGF) receptor and dephosphorylation of the tight junction occludin. This occurred in response to administration of the hydrophobic bile acids cholic acid, chenodeoxycholic acid and deoxycholic acid, but not the hydrophilic bile acid ursodeoxycholic acid [191]. These findings suggest that the effect of bile acids may be to some extent dependent on their chemical and physical properties, which in turn, relies upon microbial-mediated modification of these compounds.
–
Another factor that may influence the behavioral outcome of bile acids is the receptor that mediates the response (Table 6). Binding of the TGR5 receptor by the secondary bile acid tauroursodeoxycholic acid (TUDCA) ameliorates the depressive phenotype of CUS mice by dampening neuroinflammation (TNF-α and IL-6), as well as oxido-nitrosative and endoplasmic reticulum stress [192]. This is consistent with previous reports of the neuroprotective effects of TUDCA in microglia [193]. Additionally, some bile acids, like lithocholic acid can stimulate central PXR and vitamin D receptor (VDR) [194], which have well-established antidepressant effects [195][196]. Thus, the impact of bile acids on depressive behavior may be dependent on the specific receptor that they act upon, with FXR mediating pro-depressive phenotype, and PXR, VDR and TGR5 mediating their antidepressant action. This hypothesis has yet to be formally tested.
–
|
TABLE 6. Studies investigating the effects of bile acids on depressive-like behavior. |
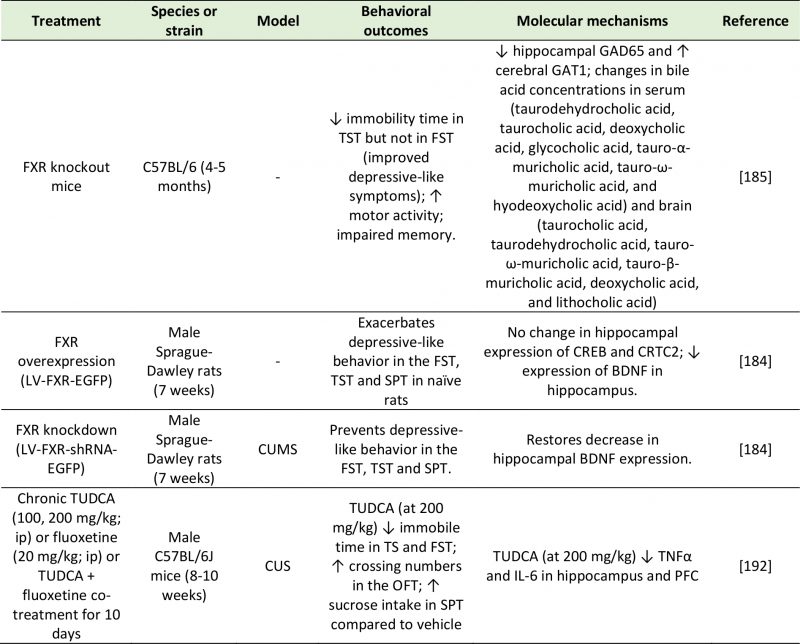 |
| BDNF: Brain-Derived Neurotrophic Factor; CREB: Camp Response Element-Binding Protein; CRTC2: CREB-Regulated Transcription Coacti-vator 2; CUMS: Chronic Unpredictable Mild Stress; CUS: Chronic Unpredictable Stress; FST: Forced-Swim Test; FXR: Farnesoid X Receptor; GAD65: Glutamic Acid Decarboxylase 65; GAT1: GABA Transporter 1; IL-6: Interleukin-6; OFT: Open Field Test; SPT: Sucrose Preference Test; Tnfα: Tumor Necrosis Factor Alpha; TST: Tail Suspension Test; TUDCA: Tauroursodeoxycholic Acid.
[184][185][192] |
–
Choline metabolites
Choline is an essential nutrient mainly obtained from dietary lecithin and carnitine, but in humans, small amounts of choline can also be synthesized in the liver [197]. Choline has structural, epigenetic and cell signaling functions. It is involved in the synthesis of acetylcholine and it is a precursor of the cell membrane components phosphatidylcholine and sphingomyelin. Although not a bacterial product per se, choline is broken down by the action the gut microbiota into a range of metabolites, including trimethylglycine (betaine) and trimethylamine (TMA). In the liver, flavin monooxygenase, a family of xenobiotic-metabolizing enzymes, can further convert TMA into trimethylamine-N-oxide (TMAO) [198]. The role of the gut microbiota in choline metabolism is demonstrated by the positive association found between the plasma levels of TMA and TMAO with the microbial order Clostridiales, the genus Ruminococcus, and the taxon Lachnospiraceae, and the negative association with proportions of S24-7, an abundant family from Bacteroidetes, in mice [199]. In a CUMS rat model, depression was associated with increased TMA but decreased TMAO levels [200]. Since choline metabolism by the gut microbiota can deplete choline stores available for the host, excessive choline-utilizing bacteria can mimic the effects of choline deficiency, such as increased occurrence of metabolic diseases, higher cardiovascular risk, as well as altered behavior [201]. For example, reduced choline availability in the hippocampus and basal ganglia was reported in MDD patients [202][203]. Reduced circulatory choline [117][204], but elevated plasma TMAO [204] were also found in patients with depressive symptoms. However, this evidence is far from conclusive, as increased central concentrations of choline have been reported in depressed adults [195][205][206] as well as children and adolescents [207][208][209]. Moreover, the choline metabolites dimethylamine, dimethylglycine, and TMAO were found to be significantly lower in the urine of MDD subject compared to controls [210]. It is apparent that contradictory evidence exists with regards to the role of these microbial metabolites in the context of depression. The finding that urinary choline concentrations were lower in moderate MDD, but higher in severe MDD compared to matched control [211] hints to the complexity of choline metabolism in relation to depressive behavior.
–
Thus, different mechanisms may exist through which choline and its metabolites influence emotional behavior. One of these potential modes of action is DNA methylation. Romano et al. [201] showed that bacterial consumption of choline reduced the availability of methyl donors and altered global DNA methylation patterns in both the adult mice and their offspring, in line with previous reports of maternal choline deficiency inducing diminished hippocampal DNA methylation and neurodevelopmental abnormalities in the offspring [212]. Choline contributes to DNA methylation by modulating the production of the methyl donor S-adenosylmethionine (SAM) [201]. In a rat model of early-life stress, supplementation of choline and betaine and other methyl donors was successful in reversing depressive-like behavior [213]. In humans, betaine exhibited a positive effect on mood by promoting the DNA methylation of SAM: in subjects with mild MDD, adjunctive treatment of SAM with betaine showed higher antidepressant efficacy than treatment with SAM alone [214].
–
An alternative mechanism involves the modulation of neurotransmission. Oral ingestion of choline increases its concentrations in the brain [215], suggesting that dietary choline can contribute to acetylcholine synthesis. This suggests that abnormal choline metabolism may promote depressive behavior by altering the availability of choline destined for acetylcholine synthesis. In fact, the neurotransmitter acetylcholine is present in significantly higher concentrations in MDD patients than in healthy subjects [216]. Since choline can reach the CNS via active transport across the BBB [217], excessive choline in the periphery may have a significant impact on mood and behavior.
–
There remains uncertainty regarding the impact of choline metabolites on behavior (Table 7). While choline deficiency may be detrimental for mental health due to insufficient DNA methylation, excessive choline may contribute to depressive pathology by leading to enhanced acetylcholine synthesis. In addition, the extent to which the gut microbiota impacts on choline metabolism remains unknown, since clinical trials have shown that TMAO levels do not respond to prebiotic administration [218][219][220].
–
|
TABLE 7. Studies investigating the effects of choline metabolites on depressive-like behavior. |
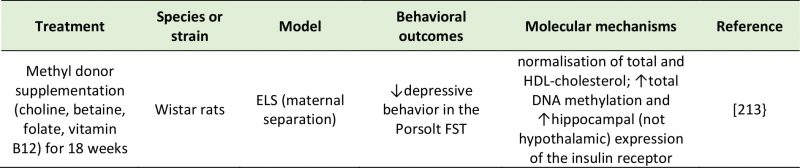 |
| HDL: High-Density Lipoprotein; FST: Forced Swim Test.
[213] |
–
Vitamins (folate)
Most bacteria in the gut, such as Lactobacillus and Bifidobacterium, synthesize vitamins (particularly B-group vitamins and vitamin K) as part of their metabolic processes in the large intestine, and humans rely heavily on the gut microbiota for their production [221]. Vitamins are essential micronutrients with ubiquitous roles in a great number of physiological processes in several organs in the human body, including the brain. Fat-soluble vitamins (such as vitamins A, D, E, and K) make up the cell membrane, while water-soluble vitamins (including the vitamin B family and vitamin C) are enzymatic co-factors for a wide number of physiological reactions [221]. Active transporters are responsible for their transport across the BBB [222]. In the CNS, their role extends from energy homeostasis to neurotransmitter production [223], meaning that vitamin deficiencies can have a significant negative impact on neurological function (e.g. neural tube defects during fetal development). Folic acid, or vitamin B9, is a vitamin of microbial origin that has been extensively implicated in the pathology of depression (Table 8), with one third of depressed patients exhibiting a folate deficiency [224]. Its biosynthesis by the gut microbiota requires the C-N binding of 6-hydroxymethyl-7,8-dihydropterin pyrophosphate (DHPPP) – obtained from guanosine triphosphate (GTP) – and p-aminobenzoic acid (pABA) – a product of the pentose phosphate pathway [225].
–
|
TABLE 8. Studies investigating the effects of folate on depressive-like behavior. |
 |
| 5-HT: 5-Hydroxytryptamine; BDNF: Brain-Derived Neurotrophic Factor; CUMS: Chronic Unpredictable Mild Stress; ELS: Early-Life Stres; FST: Forced-Swim Test; GluR1: Glutamate Receptor 1; HDL: High-Density Lipoprotein; MK-801: Non-Competitive NMDA Receptor Antagonist; OFT: Open Field Test; PCPA: Para-Chlorophenylalanine; TST: Tail Suspension Test; WAY100635: 5-HT₁A Receptor Antagonist And Full D₄ Receptor Agonist.
[213][226][227][228][232][234] |
–
Folate has an established antidepressant effect in animal models of depression [226][227][228], with some clinical studies suggesting its potential as antidepressant augmentation therapy in humans [229][230]. Using a series of pharmacological inhibitors, Brocardo et al. [226] showed that the antidepressant effects of folic acid were dependent of serotonergic (5-HT1A and 5-HT2A/2C receptors) and noradrenergic (α1- and α2-adrenoceptors) activity in mice. The finding that serotonergic and noradrenergic antagonists prevented the antidepressant effects of folic acid supports the possibility that a mechanism of action is represented by an enhancement of monoaminergic production. Folic acid can synthesize tetrahydrobiopterin (BH4), which in turns act as a cofactor for the conversion of phenylalanine and tryptophan into the neurotransmitters dopamine, norepinephrine, and 5-HT [231]. With a similar design, the same group demonstrated that the antidepressant action of folic acid was mediated by the opioid system, as treatment of the mice with different opioid receptor antagonists prevented the folate-induced reduction in immobility time in the forced swim test [232]. The authors also proposed that the action of folic acid may involve inhibition of NMDA receptors [232].
–
In addition to increased central 5-HT concentrations, folic acid can also induce an increase in BDNF and GluR1 expression in the hippocampus and association cortex, concurrent with a normalization in serum corticosterone concentration, mitochondria structure and spine synapse numbers that were altered in the CUMS model of depression [227]. Due to its involvement in the synthesis of DNA, RNA and proteins and in DNA methylation reactions [233], folate may exert these changes via epigenetics mechanisms. A diet rich in methyl donors such as folic acid has beneficial effects on exploratory behavior, social interaction and depressive-like behavior in rats [213][234]. The active metabolite of folate, 5-methyltetrahydrofolate (5-MTHF), converts homocysteine into methionine, which is used for the production of the methyl group donor SAM. In turn, SAM has been demonstrated to have antidepressant properties [235] via DNA methylation of phospholipids [236][237], with extensive consequences on neurotransmission [238]. Despite the marked improvement in depressive behavior obtained in animal studies, clinical trials have highlighted great heterogeneity and do not provide strong evidence on the benefits of the use of folate as and adjunctive strategy for depression [239].
FUTURE DIRECTIONS
Almost one third of depressed patients do not respond to treatment long-term [240]. The known impact of the microbiome on pathways involved in depression, as well as evidence linking abnormal microbiota and depressive behavior [14], suggest that targeting the gut microbiota may be an attractive strategy to improve depression-related pathological features. A strong advantage of this approach is the accessibility of the microbiome to nutritional modulation. In fact, administration of the probiotics Bifidobacterium infantis, Lactobacillus rhamnosus, Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 have proved effective in normalizing the gut microbiome and alleviating anxiety- and depression-like symptoms in both rodents [55][58] and healthy humans [241].
–
However, the complexity of the microbiota and its biochemical exchange with the host need to be better understood before this trans-kingdom communication can be harnessed to ameliorate neurological disorders. The contradictory findings reported across several studies may be reflective of this complexity. Components of the gut microbiota are in a dynamic state of equilibrium, dependent on substrate availability, exposure to antimicrobial compounds and competition with other bacterial strains. In vitro, the production of neuroactive metabolites by probiotics can be affected by nutrient availability [61]. Similarly, an intricate interplay exists between human and bacterial metabolism, as well as among the metabolic pathways reviewed. For example, intestinal neurotransmitter production is intrinsically linked to the abundance of SCFAs and bile acids in the gut, and inflammatory molecules like nitrate promote metabolism of choline by choline-utilizing bacteria [242], suggesting that the psychotropic effect of a specific metabolite may be tightly dependent on the presence of other metabolites. Another challenge encountered by gut-brain axis research is the ability to discriminate between peripheral production of neuroactive metabolites by the gut microbiota, and host production of the same metabolites in the brain. This makes it challenging to understand the extent to which the observed effect on depressive behavior can be ascribed to gut microbial metabolism per se (as compared to host central metabolism).
CONCLUSION
MDD is a multifaceted mental disorder characterized by a dysfunction of neurochemical, neuroendocrine, immune and metabolic systems. The microbiota-gut-brain axis is a bidirectional network linking the central and enteric nervous systems through the same neural, immune and metabolic routes that are dysregulated in depression [243][244]. Therefore, gut-brain axis abnormalities in depressed patients may, at least partly, account for the symptomatic presentations of depression. This review highlights how metabolites modulated by the intestinal microbiota can influence mood through their direct action on central receptors, through activation of peripheral receptors on neural, immune or neuroendocrine pathways, and through epigenetic regulation of histone deacetylation or DNA methylation (Table 9).
–
|
TABLE 9. Effects of microbial metabolites on depressive behavior in rodent and human studies. |
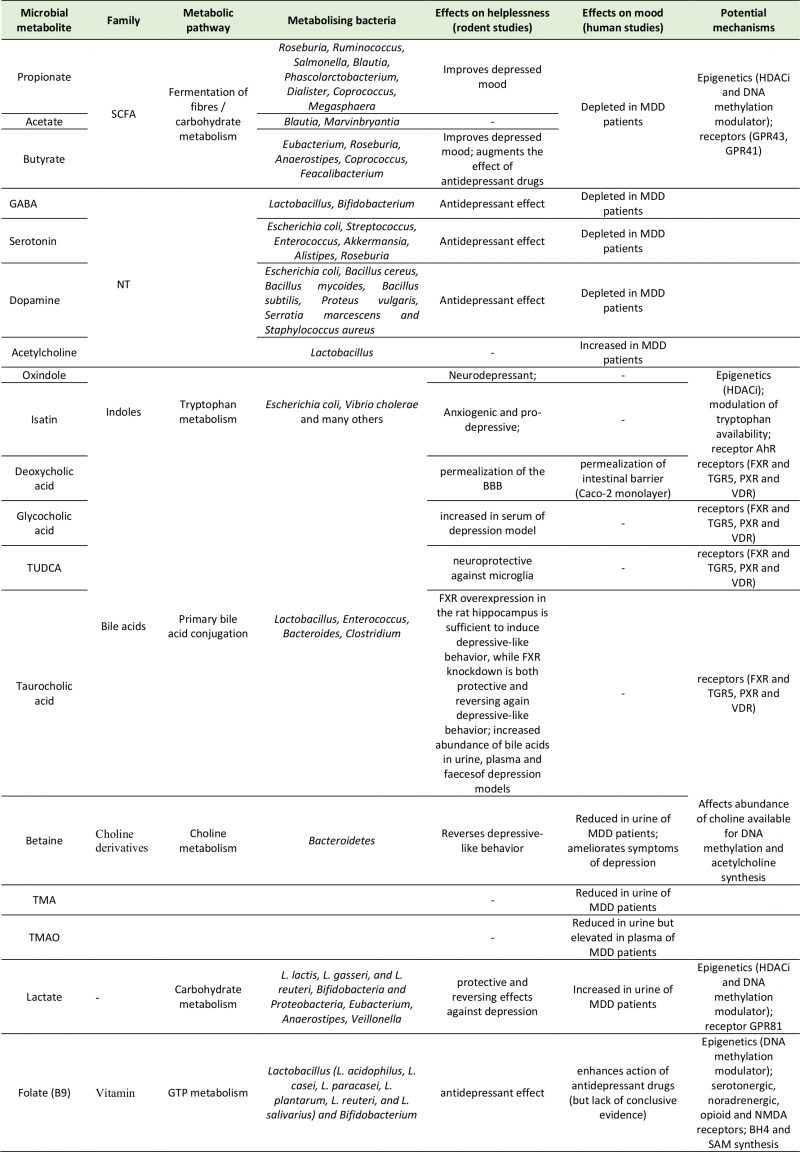 |
|
TABLE 9. Effects of microbial metabolites on depressive behavior in rodent and human studies. |
–
Addressing knowledge gaps on the multifactorial interplay between products of microbial metabolism in relation to their antidepressant effects will advance our understanding of the pathological mechanisms of depression (via the gut-brain axis) and may facilitate the development of more refined pharmacological strategies.
REFERENCES
- Culligan EP, Marchesi JR, Hill C, and Sleator RD (2012). Mining the human gut microbiome for novel stress resistance genes. Gut Microbes 3(4): 394-397. doi: 10.4161/gmic.20984
- Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, and Pettersson S (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108(7): 3047–3052. doi: 10.1073/pnas.1010529108
- Aziz Q, and Thompson DG (1998). Brain-gut axis in health and disease. Gastroenterology 114(3): 559–578. doi: 10.1016/s0016-5085(98)70540-2
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, and Cryan JF (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20(9): 509–518. doi: 10.1016/j.molmed.2014.05.002
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, and Koga Y (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558(1): 263–275. doi: 10.1113/jphysiol.2004.063388
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association.
- Chung YCE, Chen HC, Chou HCL, Chen IM, Lee MS, Chuang LC, Liu YW, Lu ML, Chen CH, Wu CH, Huang MC, Liao SC, Ni YH, Lai MS, Shih WL, and Kuo PH (2019). Exploration of microbiota targets for major depressive disorder and mood related traits. J Psychiatr Res 111: 74–82. doi: 10.1016/j.jpsychires.2019.01.016
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, and Ruan B (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48: 186–194. doi: 10.1016/j.bbi.2015.03.016
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, and Rudi K (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26(8): 1155–1162. doi: 10.1111/nmo.12378
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, and Xie P (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21(6): 786–796. doi: 10.1038/mp.2016.44
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, and Raes J (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4(4):623-632. doi: 10.1038/s41564-018-0337-x
- Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, and Bailey MT (2014). Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol 14:189. doi: 10.1186/1471-2180-14-189
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, and Rabot S (2014). Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42:207-17. doi: 10.1016/j.psyneuen.2014.01.014
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, and Dinan TG (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82: 109–118. doi: 10.1016/j.jpsychires.2016.07.019
- Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, Li G, Yuan Y, Wang J, Zhang Y, Zhao L, Zhang M, Kang Y, and Liang Y (2018). Possible association of firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr Dis Treat 14:3329-3337. doi: 10.2147/NDT.S188340
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, and Cryan JF (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277: 32–48. doi: 10.1016/j.bbr.2014.07.027
- Heijtz Diaz R, Wang S, Anuard F, Qian Y, Björkholmd B, Samuelssond A, Hibberdc ML, Forssberg H, and Pettersson S (2011). Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108(7): 3047–3052. doi: 10.1073/pnas.1010529108
- Neufeld KM, Kang N, Bienenstock J, and Foster JA (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23(3): 255-64. doi: 10.1111/j.1365-2982.2010.01620.x
- Hoban AE, Moloney RD, Golubeva A V., McVey Neufeld KA, O’Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, and Cryan JF (2016). Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 339: 463–477. doi: 10.1016/j.neuroscience.2016.10.003
- Ressler KJ, and Mayberg HS (2007). Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci 10(9):1116-24. doi: 10.1038/nn1944
- Luheshi GN, Bluthé RM, Rushforth D, Mulcahy N, Konsman JP, Goldbach M, and Dantzer R (2000). Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton Neurosci 85(1-3):127-32. doi: 10.1016/S1566-0702(00)00231-9
- Konsman JP, Luheshi GN, Bluthé RM, and Dantzer R (2000). The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci 12(12):4434-46. doi: 10.1046/j.0953-816X.2000.01319.x
- Caspani G, Corbet Burcher G, Garralda ME, Cooper M, Pierce CM, Als LC, and Nadel S (2018). Inflammation and psychopathology in children following PICU admission: An exploratory study. Evid Based Ment Health 21(4):139-144. doi: 10.1136/ebmental-2018-300027
- Fung TC, Olson CA, and Hsiao EY (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20(2): 145–155. doi: 10.1038/nn.4476
- Galland L (2014). The Gut Microbiome and the Brain. J Med Food 17(12): 1261–1272. doi: 10.1089/jmf.2014.7000
- Heumann D, Barras C, Severin A, Glauser MP, and Tomasz A (1994). Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun 62(7): 2715–2721. PMID: 7516310
- Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, Forssberg H, and Diaz Heijtz R (2017). The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 22(2): 257–266. doi: 10.1038/mp.2016.182
- Ulevitch RJ, and Tobias PS (2003). Receptor-Dependent Mechanisms of Cell Stimulation by Bacterial Endotoxin. Annu Rev Immunol 13(1): 437–457. doi: 10.1146/annurev.iy.13.040195.002253
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, and Schedlowski M (2011). Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6(12): e28330. doi: 10.1371/journal.pone.0028330
- Hoyles L, Snelling T, Umlai UK, Nicholson JK, Carding SR, Glen RC, and McArthur S (2018). Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 6(1):55. doi: 10.1186/s40168-018-0439-y
- Sternberg EM (2006). Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol 6(4):318-28. doi: 10.1038/nri1810
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, and Siuzdak G (2009). Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci 106(10): 3698–3703. doi: 10.1073/pnas.0812874106
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, and Pettersson S (2012). Host-Gut Microbiota Metabolic Interactions. Science 336(6086):1262-7. doi: 10.1126/science.1223813
- Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Thomas EL, and Bell JD (2014). The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5: 3611. doi: 10.1038/ncomms4611
- Berger M, Gray JA, and Roth BL (2009). The Expanded Biology of Serotonin. Annu Rev Med 60(1): 355–366. doi: 10.1146/annurev.med.60.042307.110802
- Hyland NP, and Cryan JF (2010). A gut feeling about GABA: Focus on GABAB receptors. Front Pharmacol 1:124. doi: 10.3389/fphar.2010.00124
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, and Stanton C (2012). γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113(2): 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
- Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, and Versalovic J (2017). GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29(1). doi: 10.1111/nmo.12904
- Cho YR, Chang JY, and Chang HC (2007). Production of γ-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from Kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol 17(1):104-9. PMID: 18051360
- Komatsuzaki N, Shima J, Kawamoto S, Momose H, and Kimura T (2005). Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22(6): 497-504. doi: 10.1016/j.fm.2005.01.002
- Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, and Gobbetti M (2007). Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73(22):7283-90. doi: 10.1128/AEM.01064-07
- Shishov VA, Kirovskaya TA, Kudrin VS, and Oleskin A V. (2009). Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Appl Biochem Microbiol 45(5): 494-497. doi: 10.1134/s0003683809050068
- Tsavkelova E, Botvinko I, Kudrin V, and Oleskin A (2000). Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem 372(1–6): 115–7. PMID: 10935181
- Stanaszek PM, Snell JF, and O’Neill JJ (1977). Isolation, extraction, and measurement of acetylcholine from Lactobacillus plantarum. Appl Environ Microbiol 34(2):237-9. PMID: 907345
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, and Sudo N (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol – Gastrointest Liver Physiol 303: G1288-95. doi: 10.1152/ajpgi.00341.2012
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, and Benno Y (2013). Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci 7: 9. doi: 10.3389/fnsys.2013.00009
- Sampson TR, and Mazmanian SK (2015). Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17:565–576. doi: 10.1016/j.chom.2015.04.011
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, and Hsiao EY (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2): 264–276. doi: 10.1016/j.cell.2015.02.047
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, and Cryan JF (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18(6): 666–673. doi: 10.1038/mp.2012.77
- Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, and Stanisz GJ (2016). Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 125: 988–995. doi: 10.1016/j.neuroimage.2015.11.018
- Ko CY, Lin HTV, and Tsai GJ (2013). Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem 48(4): 559–568. doi: 10.1016/j.procbio.2013.02.021
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Orešič M, and Bäckhed F (2010). The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 51(5): 1101–1112. doi: 10.1194/jlr.M002774
- Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, and Benno Y (2012). Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep 2: 233. doi: 10.1038/srep00233
- Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, and Mazmanian SK (2014). Specialized metabolites from the microbiome in health and disease. Cell Metab 20:719–730. doi: 10.1016/j.cmet.2014.10.016
- Bravo JA, Forsythe P, Chew M V., Escaravage E, Savignac HM, Dinan TG, Bienenstock J, and Cryan JF (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 108(38): 16050–16055. doi: 10.1073/pnas.1102999108
- Baganz NL, and Blakely RD (2013). A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci 4(1):48–63. doi: 10.1021/cn300186b
- Auteri M, Zizzo MG, and Serio R (2015). GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93: 11–21. doi: 10.1016/j.phrs.2014.12.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, and Dinan TG (2008). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res 43(2): 164–174. doi: 10.1016/j.jpsychires.2008.03.009
- Breit S, Kupferberg A, Rogler G, and Hasler G (2018). Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry 9:44. doi: 10.3389/fpsyt.2018.00044
- Strandwitz P (2018). Neurotransmitter modulation by the gut microbiota. Brain Res 1693:128–133. doi: 10.1016/j.brainres.2018.03.015
- Lyte M (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 33(8): 574–581. doi: 10.1002/bies.201100024
- Bergman EN (2017). Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70(2): 567–590. doi: 10.1152/physrev.1990.70.2.567
- van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, and Cryan JF (2018). Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol 596(20): 4923–4944. doi: 10.1113/JP276431
- Chen JJ, Zhou CJ, Liu Z, Fu YY, Zheng P, Yang DY, Li Q, Mu J, Wei YD, Zhou JJ, Huang H, and Xie P (2015). Divergent Urinary Metabolic Phenotypes between Major Depressive Disorder and Bipolar Disorder Identified by a Combined GC-MS and NMR Spectroscopic Metabonomic Approach. J Proteome Res 14(8): 3382–3389. doi: 10.1021/acs.jproteome.5b00434
- Skonieczna-żydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, Łoniewski I, Kaczmarczyk M, Marlicz W, Czerwińska-Rogowska M, Pełka-Wysiecka J, Dec K, and Stachowska E (2018). Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients 10(12): E1939. doi: 10.3390/nu10121939
- Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, and Cryan JF (2016). The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int 99:110–132. doi: 10.1016/j.neuint.2016.06.011
- Cani PD, and Knauf C (2016). How gut microbes talk to organs: The role of endocrine and nervous routes. Mol Metab 5(9):743–752. doi: 10.1016/j.molmet.2016.05.011
- Bourassa MW, Alim I, Bultman SJ, and Ratan RR (2016). Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett 625:56–63. doi: 10.1016/j.neulet.2016.02.009
- Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal K V., Poulsen SS, Han S, Jones RM, Offermanns S, and Schwartz TW (2013). GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154(10): 3552–3564. doi: 10.1210/en.2013-1142
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, and Gordon JI (2008). Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci 105(43): 16767–16772. doi: 10.1073/pnas.0808567105
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, and Gribble FM (2012). Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61(2): 364–371. doi: 10.2337/db11-1019
- Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, and Cani PD (2014). Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 8(10): 2116–2130. doi: 10.1038/ismej.2014.45
- Priori D, Colombo M, Clavenzani P, Jansman AJM, Lallès JP, Trevisi P, and Bosi P (2015). The olfactory receptor OR51E1 is present along the gastrointestinal tract of pigs, co-localizes with enteroendocrine cells and is modulated by intestinal microbiota. PLoS One 10(6): e0129501. doi: 10.1371/journal.pone.0129501
- López Soto EJ, Gambino LO, and Mustafá ER (2014). Free fatty acid receptor 3 is a key target of short chain fatty acid. Channels 8(3): 169–171. doi: 10.4161/chan.28956
- Nøhr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, and Møller M (2015). Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290: 126–137. doi: 10.1016/j.neuroscience.2015.01.040
- Goswami C, Iwasaki Y, and Yada T (2018). Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem 57: 130–135. doi: 10.1016/j.jnutbio.2018.03.009
- Karuri AR, Dobrowsky E, and Tannock IF (1993). Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br J Cancer 68(6): 1080–1087. doi: 10.1038/bjc.1993.485
- Vijay N, and Morris M (2014). Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr Pharm Des 20(10): 1487–1498. doi: 10.2174/13816128113199990462
- Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, Tedford C, Fitzpatrick J, Irani C, Busza A, Garcia-Perez I, Fountana S, Holmes E, Goldstone AP, and Frost GS (2016). Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr 104(1): 5–14. doi: 10.3945/ajcn.115.126706
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu D V., Rauch SL, and Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166(6): 702–710. doi: 10.1176/appi.ajp.2008.08081201
- Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, and Vinolo MAR (2016). Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol 5(4):e73. doi: 10.1038/cti.2016.17
- Erny D, De Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, Mccoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, and Prinz M (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7): 965–977. doi: 10.1038/nn.4030
- Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, and Salminen A (2004). Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol 141(5): 874–880. doi: 10.1038/sj.bjp.0705682
- Tedelind S, Westberg F, Kjerrulf M, and Vidal A (2007). Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J Gastroenterol 13(20): 2826–2832. doi: 10.3748/wjg.v13.i20.2826
- MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, Taylor AR, Kavaliers M, and Ossenkopp KP (2007). Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res 176(1): 149–169. doi: 10.1016/j.bbr.2006.07.025
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, and Marsland BJ (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20(2): 159–166. doi: 10.1038/nm.3444
- Kim HJ, Leeds P, and Chuang DM (2009). The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem 110(4): 1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x
- Yoo DY, Kim W, Nam SM, Kim DW, Chung JY, Choi SY, Yeo Sung Yoon, Won MH, and Hwang IK (2011). Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res 36(10): 1850–1857. doi: 10.1007/s11064-011-0503-5
- MacFabe DF, Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, and Possmayer F (2010). Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: Further development of a potential model of autism spectrum disorders. J Neurochem 113(2): 515–529. doi: 10.1111/j.1471-4159.2010.06614.x
- Frye RE, Sequeira JM, Quadros E V., James SJ, and Rossignol DA (2013). Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry 18(3): 369–381. doi: 10.1038/mp.2011.175
- Wei Y Bin, Melas PA, Wegener G, Mathe AA, and Lavebratt C (2015). Antidepressant-like effect of sodium butyrate is associated with an increase in tet1 and in 5-hydroxymethylation levels in the BDNF gene. Int J Neuropsychopharmacol 18(2): 1–10. doi: 10.1093/ijnp/pyu032
- Misztak P, Pańczyszyn-Trzewik P, and Sowa-Kućma M (2018). Histone deacetylases (HDACs) as therapeutic target for depressive disorders. Pharmacol Reports 70(2): 398–408. doi: 10.1016/j.pharep.2017.08.001
- Davie JR (2018). Inhibition of Histone Deacetylase Activity by Butyrate. J Nutr 133(7): 2485S–2493S. doi: 10.1093/jn/133.7.2485s
- Schroeder FA, Lin CL, Crusio WE, and Akbarian S (2007). Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol Psychiatry 62(1): 55–64. doi: 10.1016/j.biopsych.2006.06.036
- Gundersen BB, and Blendy JA (2009). Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology 57(1): 67–74. doi: 10.1016/j.neuropharm.2009.04.008
- Han A, Sung Y Bin, Chung SY, and Kwon MS (2014). Possible additional antidepressant-like mechanism of sodium butyrate: Targeting the hippocampus. Neuropharmacology 81: 292–302. doi: 10.1016/j.neuropharm.2014.02.017
- Yamawaki Y, Fuchikami M, Morinobu S, Segawa M, Matsumoto T, and Yamawaki S (2012). Antidepressant-like effect of sodium butyrate (HDAC inhibitor) and its molecular mechanism of action in the rat hippocampus. World J Biol Psychiatry 13(6): 458–467. doi: 10.3109/15622975.2011.585663
- Sarkar A, Chachra P, Kennedy P, Pena CJ, Desouza LA, Nestler EJ, and Vaidya VA (2014). Hippocampal HDAC4 contributes to postnatal fluoxetine-evoked depression-like behavior. Neuropsychopharmacology 39(9): 2221–2232. doi: 10.1038/npp.2014.73
- Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, Shirawachi S, Asano S, Aizawa H, Yamawaki S, Kanematsu T, and Akagi H (2018). Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res 1680: 13–38. doi: 10.1016/j.brainres.2017.12.004
- Sun J, Wang F, Hong G, Pang M, Xu H, Li H, Tian F, Fang R, Yao Y, and Liu J (2016). Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett 618: 159–166. doi: 10.1016/j.neulet.2016.03.003
- Nankova BB, Agarwal R, MacFabe DF, and La Gamma EF (2014). Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells – Possible relevance to autism spectrum disorders. PLoS One 9(8): e103740. doi: 10.1371/journal.pone.0103740
- Wang P, Zhang Y, Gong Y, Yang R, Chen Z, Hu W, Wu Y, Gao M, Xu X, Qin Y, and Huang C (2018). Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol Dis 111: 12–25. doi: 10.1016/j.nbd.2017.12.006
- Valvassori S, Resende W, Budni J, Dal-Pont G, Bavaresco D, Reus G, Carvalho A, Goncalves C, Furlanetto C, Streck E, and Quevedo J (2015). Sodium Butyrate, a Histone Deacetylase Inhibitor, Reverses Behavioral and Mitochondrial Alterations in Animal Models of Depression Induced by Early- or Late-life Stress. Curr Neurovasc Res 12(4): 312–320. doi: 10.2174/1567202612666150728121121
- Aoyama M, Kotani J, and Usami M (2010). Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26(6): 653–661. doi: 10.1016/j.nut.2009.07.006
- Li J, Hou L, Wang C, Jia X, Qin X, and Wu C (2018). Short Term Intrarectal Administration of Sodium Propionate Induces Antidepressant-Like Effects in Rats Exposed to Chronic Unpredictable Mild Stress. Front Psychiatry 9: 454. doi: 10.3389/fpsyt.2018.00454
- Teraishi T, Hori H, Sasayama D, Matsuo J, Ogawa S, Ota M, Hattori K, Kajiwara M, Higuchi T, and Kunugi H (2015). 13 C-tryptophan breath test detects increased catabolic turnover of tryptophan along the kynurenine pathway in patients with major depressive disorder. Sci Rep 5: 15994. doi: 10.1038/srep15994
- El-Ansary AK, Bacha AB, and Kotb M (2012). Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation 9: 74. doi: 10.1186/1742-2094-9-74
- Kanski R, Sneeboer MAM, van Bodegraven EJ, Sluijs JA, Kropff W, Vermunt MW, Creyghton MP, De Filippis L, Vescovi A, Aronica E, van Tijn P, van Strien ME, and Hol EM (2014). Histone acetylation in astrocytes suppresses GFAP and stimulates a reorganization of the intermediate filament network. J Cell Sci 127(20): 4368–4380. doi: 10.1242/jcs.145912
- Segain JP, Galmiche JP, Raingeard De La Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, and Blottière HM (2000). Butyrate inhibits inflammatory responses through NFκB inhibition: Implications for Crohn’s disease. Gut 47(3): 397–403. doi: 10.1136/gut.47.3.397
- Patnala R, Arumugam T V., Gupta N, and Dheen ST (2017). HDAC Inhibitor Sodium Butyrate-Mediated Epigenetic Regulation Enhances Neuroprotective Function of Microglia During Ischemic Stroke. Mol Neurobiol 54(8): 6391–6411. doi: 10.1007/s12035-016-0149-z
- Wang J, Wei Z, Zhang X, Wang Y, Yang Z, and Fu Y (2017). Propionate protects against lipopolysaccharide-induced mastitis in mice by restoring blood-milk barrier disruption and suppressing inflammatory response. Front Immunol 8: 1108. doi: 10.3389/fimmu.2017.01108
- De Almeida LMV, Funchal C, Gottfried C, Wajner M, and Pessoa-Pureur R (2006). Propionic acid induces cytoskeletal alterations in cultured astrocytes from rat cerebral cortex. Metab Brain Dis 21(1): 51–62. doi: 10.1007/s11011-006-9002-9
- MacFabe DF (2012). Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Heal Dis 23. doi: 10.3402/mehd.v23i0.19260
- Shultz SR, MacFabe DF, Martin S, Jackson J, Taylor R, Boon F, Ossenkopp KP, and Cain DP (2009). Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: Further development of a rodent model of autism. Behav Brain Res 200(1): 33–41. doi: 10.1016/j.bbr.2008.12.023
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, and Cryan JF (2017). Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry 82(7): 472–487. doi: 10.1016/j.biopsych.2016.12.031
- Gao X, Zheng X, Li Z, Zhou Y, Sun H, Zhang L, Guo X, Du G, and Qin X (2011). Metabonomic study on chronic unpredictable mild stress and intervention effects of Xiaoyaosan in rats using gas chromatography coupled with mass spectrometry. J Ethnopharmacol 137(1):690-9. doi: 10.1016/j.jep.2011.06.024
- Liu CC, Wu YF, Feng GM, Gao XX, Zhou YZ, Hou WJ, Qin XM, Du GH, and Tian JS (2015). Plasma-metabolite-biomarkers for the therapeutic response in depressed patients by the traditional Chinese medicine formula Xiaoyaosan: A1H NMR-based metabolomics approach. J Affect Disord 185: 156–163. doi: 10.1016/j.jad.2015.05.005
- Primec M, Mičetić-Turk D, and Langerholc T (2017). Analysis of short-chain fatty acids in human feces: A scoping review. Anal Biochem 526:9-21. doi: 10.1016/j.ab.2017.03.007
- Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, Verdu EF, Collins SM, and Bercik P (2018). High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6(1):57. doi: 10.1186/s40168-018-0433-4
- Rooks MG, and Garrett WS (2016). Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16(6):341-52. doi: 10.1038/nri.2016.42
- Chambers ES, Morrison DJ, and Frost G (2015). Control of appetite and energy intake by SCFA: What are the potential underlying mechanisms? Proc Nutr Soc 74(3):328-36. doi: 10.1017/S0029665114001657
- Comalada M, Bailón E, De Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, and Gálvez J (2006). The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol 132(8):487-97. doi: 10.1007/s00432-006-0092-x
- Pluznick JL (2013). A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5(2):202-7. doi: 10.4161/gmic.27492
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, and Bakker BM (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54(9):2325-40. doi: 10.1194/jlr.r036012
- Morrison DJ, and Preston T (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7(3):189-200. doi: 10.1080/19490976.2015.1134082
- Pan X, Chen F, Wu T, Tang H, and Zhao Z (2009). Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B 10(4):258-63. doi: 10.1631/jzus.b0820261
- Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, Kitzman DW, Becton T, Read R, and Yadav H (2018). Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep 8(1):12649. doi: 10.1038/s41598-018-30114-4
- Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, and Dougherty DM (2009). L-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res 2:45-60. doi: 10.4137/ijtr.s2129
- Young SN (2013). Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J Psychiatry Neurosci 38(5):294-305. doi: 10.1503/jpn.120209
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, and Diksic M (1997). Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci 94(10):5308-13. doi: 10.1073/pnas.94.10.5308
- Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M, Doucette JT, Alonso A, Collins KA, Neumeister A, and Charney DS (2011). Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry 69(8):804-7. doi: 10.1016/j.biopsych.2010.12.033
- Van Der Veen FM, Evers EAT, Deutz NEP, and Schmitt JAJ (2007). Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology 32(1):216-24. doi: 10.1038/sj.npp.1301212
- Smith KA, Fairburn CG, and Cowen PJ (1997). Relapse of depression after vapid depletion of tryptophan. Lancet 349(9056):915-9. doi: 10.1016/S0140-6736(96)07044-4
- Moreno FA, Gelenberg AJ, Heninger GR, Potter RL, McKnight KM, Allen J, Phillips AP, and Delgado PL (1999). Tryptophan depletion and depressive vulnerability. Biol Psychiatry 46(4):498-505. doi: 10.1016/S0006-3223(99)00095-5
- Booij L, Van Der Does AJW, Haffmans PMJ, Riedel WJ, Fekkes D, and Blum MJB (2005). The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. J Psychopharmacol 19(3):267-75. doi: 10.1177/0269881105051538
- Booij L, Van Der Does AJW, Haffmans PMJ, and Riedel WJ (2005). Acute tryptophan depletion in depressed patients treated with a selective serotonin-noradrenalin reuptake inhibitor: Augmentation of antidepressant response? J Affect Disord 86(2-3):305-11. doi: 10.1016/j.jad.2005.01.012
- Delgado PL, Price LH, Miller HL, Salomon RM, Licinio J, Krystal JH, Heninger GR, and Charney DS (1991). Rapid serotonin depletion as a provocative challenge test for patients with major depression: relevance to antidepressant action and the neurobiology of depression. Psychopharmacol Bull 27(3):321-30. PMID: 1775606.
- Müller N, and Schwarz MJ (2008). A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: Schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci 258 (Suppl 2):97-106. doi: 10.1007/s00406-008-2012-3
- Melillo G, Cox GW, Biragyn A, Sheffler LA, and Varesio L (1994). Regulation of nitric-oxide synthase mRNA expression by interferon-γ and picolinic acid. J Biol Chem 269(11):8128-33. PMID: 7510678
- Oxenkrug G (2005). Antioxidant effects of N-acetylserotonin: Possible mechanisms and clinical implications. Ann N Y Acad Sci 1053:334-47. doi: 10.1196/annals.1344.029
- Lapin IP, and Oxenkrug GF (1969). Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1(7586):132-6. doi: 10.1016/s0140-6736(69)91140-4
- Pardridge WM. (1979). The role of blood-brain barrier transport of tryptophan and other neutral amino acids in the regulation of substrate-limited pathways of brain amino acid metabolism. J Neural Transm Suppl 15: 43–54. doi: 10.1007/978-3-7091-2243-3_4
- Li G, and Young KD (2013). Indole production by the tryptophanase TnaA in escherichia coli is determined by the amount of exogenous tryptophan. Microbiol 159(Pt 2):402-10. doi: 10.1099/mic.0.064139-0
- Lee JH, and Lee J (2010). Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34(4):426-44. doi: 10.1111/j.1574-6976.2009.00204.x
- Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, and Reimann F (2014). Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep 9(4):1202-8. doi: 10.1016/j.celrep.2014.10.032
- Bansal T, Alaniz RC, Wood TK, and Jayaraman A (2010). The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci 107(1):228-33: doi: 10.1073/pnas.0906112107
- Carpenedo R, Mannaioni G, and Moroni F (2002). Oxindole, a Sedative Tryptophan Metabolite, Accumulates in Blood and Brain of Rats with Acute Hepatic Failure. J Neurochem 70(5):1998-2003. doi: 10.1046/j.1471-4159.1998.70051998.x
- Bhattacharya SK, Ramnathan M, and Glover V (2000). Intraventricular administration of isatin in rats: antidiuretic, dipsogenic, anorexiant and emetic effects. Biog Amin 16(1):63-71.
- Satayan KS, Rai A, Jaiswal AK, Acharya SB, and Bhattacharya SK (1995). Isatin, a putative anxiogenic endocoid, induces memory dysfunction in rats. Indian J Exp Biol 33(8):576-9. PMID: 8543325
- Bhattacharya SK, Mitra SK, and Acharya SB (1991). Anxiogenic activity of isatin, a putative biological factor, in rodents. J Psychopharmacol 5(3):202-6. doi: 10.1177/026988119100500304
- Abel EL (1995). Behavioral effects of isatin on open field activity and immobility in the forced swim test in rats. Physiol Behav 57(3):611-3. doi: 10.1016/0031-9384(94)00365-C
- Kochanowska-Karamyan AJ, and Hamann MT (2010). Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem Rev 110(8):4489-97. doi: 10.1021/cr900211p
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, and Quintana FJ (2016). Type i interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22(6): 586–597. doi: 10.1038/nm.4106
- Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, Daugé V, Maguin E, Naudon L, and Rabot S (2018). Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci 12:216. doi: 10.3389/fnins.2018.00216
- Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, and Sokol H (2016). CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22(6):598-605. doi: 10.1038/nm.4102
- Zelante T, Iannitti RG, Cunha C, DeLuca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, and Romani L (2013). Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39(2):372-85. doi: 10.1016/j.immuni.2013.08.003
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, and Mani S (2014). Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 41(2):296-310. doi: 10.1016/j.immuni.2014.06.014
- Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, Jeong YG, Lee YL, Kim YM, Kwon YG, and Won MH (2009). Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res 87(9):2126-37. doi: 10.1002/jnr.22030
- Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, and Pappolla MA (1999). Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem 274(31):21937-42. doi: 10.1074/jbc.274.31.21937
- Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, and Brunet P (2007). The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5(6):1302-8. doi: 10.1111/j.1538-7836.2007.02540.x
- Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, De los Reyes-Gavilán CG, and Salazar N (2016). Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7: 185. doi: 10.3389/fmicb.2016.00185
- Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S, and Shibata S (2018). Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci Rep 8(1): 1395. doi: 10.1038/s41598-018-19836-7
- Knudsen GM, Paulson OB, and Hertz MM (1991). Kinetic analysis of the human blood-brain barrier transport of lactate and its influence by hypercapnia. J Cereb Blood Flow Metab 11(4): 581–586. doi: 10.1038/jcbfm.1991.107
- Walls AB, Heimbürger CM, Bouman SD, Schousboe A, and Waagepetersen HS (2009). Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience 158(1): 284–292. doi: 10.1016/j.neuroscience.2008.09.058
- Barros LF (2013). Metabolic signaling by lactate in the brain. Trends Neurosci 36(7): 396–404. doi: 10.1016/j.tins.2013.04.002
- Mosienko V, Teschemacher AG, and Kasparov S (2015). Is L-lactate a novel signaling molecule in the brain? J Cereb Blood Flow Metab 35(7):1069–1075. doi: 10.1038/jcbfm.2015.77
- Chen J jun, Zhou C juan, Zheng P, Cheng K, Wang H yang, Li J, Zeng L, and Xie P (2017). Differential urinary metabolites related with the severity of major depressive disorder. Behav Brain Res 332: 280–287. doi: 10.1016/j.bbr.2017.06.012
- Swann JR, Garcia-Perez I, Braniste V, Wilson ID, Sidaway JE, Nicholson JK, Pettersson S, and Holmes E (2017). Application of1H NMR spectroscopy to the metabolic phenotyping of rodent brain extracts: A metabonomic study of gut microbial influence on host brain metabolism. J Pharm Biomed Anal 143: 141–146. doi: 10.1016/j.jpba.2017.05.040
- Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, and Bergersen LH (2014). Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24(10): 2784–2795. doi: 10.1093/cercor/bht136
- Shoblock J, Welty N, Chen G, Yun S, Lovenberg T, Liu C, Bonaventure P, and Shelton J (2012). Characterizing the Behavioral Phenotype of GPR81 Knockout Mice: Is GPR81 a Novel Target Relevant to Mood Disorders? 67th Annual Scientific Convention and Meeting of the Society-of-Biological-Psychiatry, p. 145S.
- Hoque R, Farooq A, Ghani A, Gorelick F, and Mehal WZ (2014). Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via gpr81-mediated suppression of innate immunity. Gastroenterology. 146(7): 1763–1774. doi: 10.1053/j.gastro.2014.03.014
- Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, and Bergersen LH (2015). The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J Neurosci Res 93(7): 1045–1055. doi: 10.1002/jnr.23593
- Karnib N, El-Ghandour R, El Hayek L, Nasrallah P, Khalifeh M, Barmo N, Jabre V, Ibrahim P, Bilen M, Stephan JS, Holson EB, Ratan RR, and Sleiman SF (2019). Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 44(6):1152-1162. doi: 10.1038/s41386-019-0313-z
- Carrard A, Elsayed M, Margineanu M, Boury-Jamot B, Fragnière L, Meylan EM, Petit JM, Fiumelli H, Magistretti PJ, and Martin JL (2018). Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry 23(2): 392–399. doi: 10.1038/mp.2016.179
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, and Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810-23. doi: 10.1016/j.cell.2011.02.018
- Petriz BA, Castro AP, Almeida JA, Gomes CPC, Fernandes GR, Kruger RH, Pereira RW, and Franco OL (2014). Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics 15: 511. doi: 10.1186/1471-2164-15-511
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, and Woods JA (2018). Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc 50(4):747-757. doi: 10.1249/MSS.0000000000001495
- Russell DW (2003). The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu Rev Biochem 72(1): 137–174. doi: 10.1146/annurev.biochem.72.121801.161712
- Ferrebee CB, and Dawson PA (2015). Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm Sin B 5(2):129–134. doi: 10.1016/j.apsb.2015.01.001
- Mertens KL, Kalsbeek A, Soeters MR, and Eggink HM (2017). Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci 11:617. doi: 10.3389/fnins.2017.00617
- Ding L, Yang L, Wang Z, and Huang W (2015). Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 5(2):135-44. doi: 10.1016/j.apsb.2015.01.004
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B, and Kemper JK (2014). Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516(729): 108–111. doi: 10.1038/nature13949
- Huang C, Wang J, Hu W, Wang C, Lu X, Tong L, Wu F, and Zhang W (2016). Identification of functional farnesoid X receptors in brain neurons. FEBS Lett 590(18):3233–3242. doi: 10.1002/1873-3468.12373
- Chen WG, Zheng JX, Xu X, Hu YM, and Ma YM (2018). Hippocampal FXR plays a role in the pathogenesis of depression: A preliminary study based on lentiviral gene modulation. Psychiatry Res 264: 374–379. doi: 10.1016/j.psychres.2018.04.025
- Huang F, Wang T, Lan Y, Yang L, Pan W, Zhu Y, Lv B, Wei Y, Shi H, Wu H, Zhang B, Wang J, Duan X, Hu Z, and Wu X (2015). Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci 9: 70. doi: 10.3389/fnbeh.2015.00070
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, and Gonzalez FJ (2000). Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102(6): 731–744. doi: 10.1016/S0092-8674(00)00062-3
- Zhang F, Jia Z, Gao P, Kong H, Li X, Lu X, Wu Y, and Xu G (2010). Metabonomics study of urine and plasma in depression and excess fatigue rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Mol Biosyst 6(5): 852. doi: 10.1039/b914751a
- Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, and Zou Z (2017). Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 138: 231–239. doi: 10.1016/j.jpba.2017.02.008
- Su ZH, Jia HM, Zhang HW, Feng YF, An L, and Zou ZM (2014). Hippocampus and serum metabolomic studies to explore the regulation of Chaihu-Shu-Gan-San on metabolic network disturbances of rats exposed to chronic variable stress. Mol Biosyst 10(3): 549–561. doi: 10.1039/c3mb70377k
- Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, and DeMorrow S (2014). Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis 46(6): 527–534. doi: 10.1016/j.dld.2014.01.159
- Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, and Paludetto R (2008). Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Liver Physiol 294(4): G906–G913. doi: 10.1152/ajpgi.00043.2007
- Lu X, Yang RR, Zhang JL, Wang P, Gong Y, Hu W feng, Wu Y, Gao M hui, and Huang C (2018). Tauroursodeoxycholic acid produces antidepressant-like effects in a chronic unpredictable stress model of depression via attenuation of neuroinflammation, oxido-nitrosative stress, and endoplasmic reticulum stress. Fundam Clin Pharmacol 32(4): 363–377. doi: 10.1111/fcp.12367
- Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, and Romero-Ramírez L (2017). TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J Cell Physiol 232(8): 2231–2245. doi: 10.1002/jcp.25742
- Hylemon PBPB, Zhou H, Pandak WMWM, Ren S, Gil G, and Dent P (2009). Bile acids as regulatory molecules. J Lipid Res 50(8): 1509–20. doi: 10.1194/jlr.R900007-JLR200
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, and Kliewer SA (2000). St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A 97(13): 7500–2. doi: 10.1073/pnas.130155097
- Spedding S (2014). Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 6(4):1501–1518. doi: 10.3390/nu6041501
- Corbin KD, and Zeisel SH (2012). Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 28(2):159-65. doi: 10.1097/MOG.0b013e32834e7b4b
- Dumas M-E, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, and Nicholson JK (2006). Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci 103(33): 12511–12516. doi: 10.1073/pnas.0601056103
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, Didonato AJ, Fu X, Hazen JE, Krajcik D, Didonato JA, Lusis AJ, and Hazen SL (2015). Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 163(7): 1585–1595. doi: 10.1016/j.cell.2015.11.055
- Tian JS, Shi BY, Xiang H, Gao S, Qin XM, and Du GH (2013). 1H-NMR-Based Metabonomic Studies on the Anti-Depressant Effect of Genipin in the Chronic Unpredictable Mild Stress Rat Model. PLoS One 8(9): e75721. doi: 10.1371/journal.pone.0075721
- Romano KA, Martinez-del Campo A, Kasahara K, Chittim CL, Vivas EI, Amador-Noguez D, Balskus EP, and Rey FE (2017). Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 22(3): 279–290.e7. doi: 10.1016/j.chom.2017.07.021
- Renshaw PF, Lafer B, Babb SM, Fava M, Stoll AL, Christensen JD, Moore CM, Yurgelun-Todd DA, Bonello CM, Pillay SS, Rothschild AJ, Nierenberg AA, Rosenbaum JF, and Cohen BM (1997). Basal ganglia choline levels in depression and response to fluoxetine treatment: An in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 41(8): 837–843. doi: 10.1016/S0006-3223(96)00256-9
- Ende G, Braus DF, Walter S, Weber-Fahr W, and Henn FA (2000). The hippocampus in patients treated with electroconvulsive therapy: A proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry 57(10): 937–943. doi: 10.1001/archpsyc.57.10.937
- Tian JS, Xia XT, Wu YF, Zhao L, Xiang H, Du GH, Zhang X, and Qin XM (2016). Discovery, screening and evaluation of a plasma biomarker panel for subjects with psychological suboptimal health state using 1 H-NMR-based metabolomics profiles. Sci Rep 6: 33820. doi: 10.1038/srep33820
- Charles HC, Lazeyras F, Krishnan KRR, Boyko OB, Payne M, and Moore D (1994). Brain choline in depression: In vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry 18(7): 1121–1127. doi: 10.1016/0278-5846(94)90115-5
- Hamakawa H, Kato T, Murashita J, and Kato N (1998). Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. Eur Arch Psychiatry Clin Neurosci 248(1): 53–58. doi: 10.1007/s004060050017
- MacMaster FP, and Kusumakar V (2006). Choline in pediatric depression. McGill J Med 9(1): 24–27. PMID: 19529805
- Moore GJ, Stewart CM, Seraji-Bozorgzad N, Paulson LD, Wilds IB, and Rosenberg DR (2002). 314. Brain chemistry in pediatric depression. Biol Psychiatry 47(8): S95. doi: 10.1016/s0006-3223(00)00578-3
- Steingard RJ, Yurgelun-Todd DA, Hennen J, Moore JEC, Moore CM, Vakili K, Young AD, Katic A, Beardslee WR, and Renshaw PF (2000). Increased orbitofrontal cortex levels of choline in depressed adolescents as detected by in vivo proton magnetic resonance spectroscopy. Biol Psychiatry 48(11): 1053–1061. doi: 10.1016/S0006-3223(00)00942-2
- Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, Zhong J, Lei Y, Melgiri ND, and Xie P (2013). Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics 12(1): 207–14. doi: 10.1074/mcp.M112.021816
- Chen J jun, Zhou C juan, Zheng P, Cheng K, Wang H yang, Li J, Zeng L, and Xie P (2017). Differential urinary metabolites related with the severity of major depressive disorder. Behav Brain Res 332: 280–287. doi: 10.1016/j.bbr.2017.06.012
- Mellott TJ, Kowall NW, Lopez-Coviella I, and Blusztajn JK (2007). Prenatal choline deficiency increases choline transporter expression in the septum and hippocampus during postnatal development and in adulthood in rats. Brain Res 1151(1): 1–11. doi: 10.1016/j.brainres.2007.03.004
- Paternain L, Martisova E, Campión J, Martínez JA, Ramírez MJ, and Milagro FI (2016). Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav Brain Res 299: 51–58. doi: 10.1016/j.bbr.2015.11.031
- Di Pierro F, and Settembre R (2015). Preliminary results of a randomized controlled trial carried out with a fixed combination of S-adenosyl-L-methionine and betaine versus amitriptyline in patients with mild depression. Int J Gen Med 8: 73–78. doi: 10.2147/IJGM.S79518
- Babb SM, Ke Y, Lange N, Kaufman MJ, Renshaw PF, and Cohen BM (2004). Oral choline increases choline metabolites in human brain. Psychiatry Res – Neuroimaging. 130(1): 1–9. doi: 10.1016/S0925-4927(03)00104-5
- Mineur YS, and Picciotto MR (2010). Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31(12):580–586. doi: 10.1016/j.tips.2010.09.004
- Sawada N, Takanaga H, Matsuo H, Naito M, Tsuruo T, and Sawada Y (1999). Choline uptake by mouse brain capillary endothelial cells in culture. J Pharm Pharmacol 51(7): 847–852. doi: 10.1211/0022357991773050
- Akbarian-Moghari A, Mohtadi-Nia J, Mofid V, Homayouni-Rad A, Asghari-Jafarabadi M, Niafar M, and Ejtahed HS (2011). Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci 94(7): 3288–3294. doi: 10.3168/jds.2010-4128
- Jung SP, Lee KM, Kang JH, Yun S Il, Park HO, Moon Y, and Kim JY (2013). Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean J Fam Med 34(2): 80–89. doi: 10.4082/kjfm.2013.34.2.80
- Tripolt NJ, Leber B, Triebl A, Köfeler H, Stadlbauer V, and Sourij H (2015). Effect of Lactobacillus casei Shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: An open-label, randomized study. Atherosclerosis 242(1): 141–144. doi: 10.1016/j.atherosclerosis.2015.05.005
- Gu Q, and Li P (2016). Biosynthesis of Vitamins by Probiotic Bacteria. In: Probiotics Prebiotics Hum Nutr Heal. doi: 10.5772/63117
- Wu D, and Pardridge WM (1999). Blood-brain barrier transport of reduced folic acid. Pharm Res 16(3): 415–419. doi: 10.1023/A:1018829920158
- Kennedy DO (2016). B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 8(2):68. doi: 10.3390/nu8020068
- Miller AL (2008). The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev 13(3):216–226. PMID: 18950248
- Rossi M, Amaretti A, and Raimondi S (2011). Folate production by probiotic bacteria. Nutrients 3(1): 118-134. doi: 10.3390/nu3010118
- Brocardo PS, Budni J, Kaster MP, Santos ARS, and Rodrigues ALS (2008). Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 54(2): 464–473. doi: 10.1016/j.neuropharm.2007.10.016
- Gao L, Liu X, Yu L, Wu J, Xu M, and Liu Y (2017). Folic acid exerts antidepressant effects by upregulating brain-derived neurotrophic factor and glutamate receptor 1 expression in brain. Neuroreport 28(16): 1078–1084. doi: 10.1097/WNR.0000000000000887
- Molina-Hernández M, Téllez-Alcántara NP, Olivera-López JI, and Jaramillo MT (2011). The folic acid combined with 17-β estradiol produces antidepressant-like actions in ovariectomized rats forced to swim. Prog Neuro-Psychopharmacology Biol Psychiatry 35(1): 60–66. doi: 10.1016/j.pnpbp.2010.08.022
- Coppen A, Chaudhry S, and Swade C (1986). Folic acid enhances lithium prophylaxis. J Affect Disord 10(1): 9–13. doi: 10.1016/0165-0327(86)90043-1
- Coppen A, and Bailey J (2000). Enhancement of the antidepressant action of fluoxetine by folic acid: A randomised, placebo controlled trial. J Affect Disord 60(2): 121–130. doi: 10.1016/S0165-0327(00)00153-1
- Wolf WA, Ziaja E, Arthur RA, Anastasiadis PZ, Levine RA, and Kuhn DM (1991). Effect of Tetrahydrobiopterin on Serotonin Synthesis, Release, and Metabolism in Superfused Hippocampal Slices. J Neurochem 57(4): 1191–1197. doi: 10.1111/j.1471-4159.1991.tb08279.x
- Brocardo PS, Budni J, Lobato KR, Santos ARS, and Rodrigues ALS (2009). Evidence for the involvement of the opioid system in the antidepressant-like effect of folic acid in the mouse forced swimming test. Behav Brain Res 200(1): 122–127. doi: 10.1016/j.bbr.2009.01.004
- Crider KS, Yang TP, Berry RJ, and Bailey LB (2012). Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv Nutr 3(1): 21–38. doi: 10.3945/an.111.000992
- McCoy CR, Rana S, Stringfellow SA, Day JJ, Wyss JM, Clinton SM, and Kerman IA (2016). Neonatal maternal separation stress elicits lasting DNA methylation changes in the hippocampus of stress-reactive Wistar Kyoto rats. Eur J Neurosci 44(10): 2829–2845. doi: 10.1111/ejn.13404
- Kagan BL, Sultzer DL, Rosenlicht N, and Gerner RH (1990). Oral S-adenosylmethionine in depression: A randomized, double-blind, placebo-controlled trial. Am J Psychiatry 147(5): 591–595. doi: 10.1176/ajp.147.5.591
- Bottiglieri T, Hyland K, and Reynolds EH (1994). The Clinical Potential of Ademetionine (S-Adenosylmethionine) in Neurological Disorders. Drugs 48(2):137–152. doi: 10.2165/00003495-199448020-00002
- Young SN, and Ghadirian AM (1989). Folic acid and psychopathology. Prog Neuropsychopharmacol Biol Psychiatry 13(6):841–863. doi: 10.1016/0278-5846(89)90037-7
- Hutto BR (1997). Folate and cobalamin in psychiatric illness. Compr Psychiatry 38(6): 305–314. doi: 10.1016/S0010-440X(97)90925-1
- Roberts E, Carter B, and Young AH (2018). Caveat emptor: Folate in unipolar depressive illness, a systematic review and meta-analysis. J Psychopharmacol 32(4):377–384. doi: 10.1177/0269881118756060
- Al-Harbi KS (2012). Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer Adherence 6:369–388. doi: 10.2147/ppa.s29716
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, and Cazaubiel JM (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 105(5): 755–764. doi: 10.1017/S0007114510004319
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, and Bäumler AJ (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339(6120): 708–711. doi: 10.1126/science.1232467
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, and Martín-Santos R (2012). Interferon-Induced Depression in Chronic Hepatitis C. J Clin Psychiatry 73(08): 1128–1138. doi: 10.4088/JCP.12r07694
- McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ, Demetrashvili MF, Miller AH, and Musselman DL (2012). Neurobehavioral effects of interferon-α in patients with hepatitis-C: Symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology 37(6): 1444–1454. doi: 10.1038/npp.2011.330
ACKNOWLEDGMENTS
Giorgia Caspani is supported by the MRC (grant number MR/N014103/1). This research was conducted as part of CAN-BIND, an Integrated Discovery Program supported by the Ontario Brain Institute, which is an independent non-profit corporation, funded par-tially by the Ontario Government.
Giorgia Caspani1, Sidney Kennedy2-5, Jane A. Foster6 and Jonathan Swann1
Gut microbiome-wide association study of depressive symptoms
Abstract
Depression is one of the most poorly understood diseases due to its elusive pathogenesis. There is an urgency to identify molecular and biological mechanisms underlying depression and the gut microbiome is a novel area of interest. Here we investigate the relation of fecal microbiome diversity and composition with depressive symptoms in 1,054 participants from the Rotterdam Study cohort and validate these findings in the Amsterdam HELIUS cohort in 1,539 subjects. We identify association of thirteen microbial taxa, including genera Eggerthella, Subdoligranulum, Coprococcus, Sellimonas, Lachnoclostridium, Hungatella, Ruminococcaceae (UCG002, UCG003 and UCG005), LachnospiraceaeUCG001, Eubacterium ventriosum and Ruminococcusgauvreauiigroup, and family Ruminococcaceae with depressive symptoms. These bacteria are known to be involved in the synthesis of glutamate, butyrate, serotonin and gamma amino butyric acid (GABA), which are key neurotransmitters for depression. Our study suggests that the gut microbiome composition may play a key role in depression.
Introduction
Depression is one of the most common mental disorders experienced worldwide with an average lifetime prevalence of 11–15%1. The prevalence has doubled and, in some countries, even tripled during the COVID-19 pandemic2. Yet, depression is also one of the most common and poorly understood diseases courtesy of its elusive pathogenesis. Treatment options are sub-optimal with most antidepressants performing only marginally better than placebo3,4 with additional costs of having side effects ranging from minor cognitive complaints to even suicide5. The low to moderate heritability6 and the small effects of genetic variants (odds ratio <1.05) identified in large genome-wide association studies (GWAS) of depression7 entails the need to go beyond genetics in the search of molecular biomarkers of depression.
Evidence is accumulating that gut microbiota may influence brain activity and behavior via neural and humoral pathways8,9 and may have translational applications in the treatment of neuropsychiatric disorders10,11,12. Several animal studies suggest that gut microbiota might have impact on the neurobiological features of depression13,14,15,16,17,18,19,20,21. Fecal microbiota transplantation of either stressed or obese animals to control animals showed significant alteration of anxiety22. Kelly et al.23 showed that transferring gut microbiota of depressed human patients to germfree rats induces behavioral and physiological features characteristic of depression in the recipient animals suggesting that gut microbiota may be involved in causal pathways leading to depression. Another study showed that pre- and probiotic consumption positively affects mood and anxiety in humans24. There have been very few studies systematically exploring the association between gut microbiome and depression in humans25. Further, the existing studies are based on very small samples (<60 cases), lacking statistical power to detect robust and reproducible associations. The most recent study including 121 cases reported depletion of butyrate producing bacteria (Coprococcus and Dialister) in individuals with depression26. However, these studies did not adjust for confounders including life style factors and medication use25, which are known to modify the gut microbiome27. A parallel study investigated the association of the gut microbiome with depressive symptoms in the multiethnic HELIUS cohort comprising of six different ethnic groups. This study has identified genera/species belonging to the families Christensencellaceae, Lachnospiraceae, and Ruminococcaceae consistently associated with depressive symptoms across ethnicities, taking a wide range of confounders into account [NCOMMS-21-20669B]. Thus far, most consistent associations have been reported for genera Eggerthella, Coproccocus, Subdoligranulum, Mitsuokella, Paraprevotella, Sutterella and family Prevotellaceae28. However, the results of existing studies are conflicting with little overlap asking for larger and more carefully designed studies28.
Here, we study the effect of gut microbiome diversity and composition on depression scores in 1,133 individuals from the Rotterdam Study while controlling for lifestyle factors and medication use. The analyses were replicated in the native Dutch participants of the HELIUS cohort (N = 1,539). Finally, we performed Mendelian Randomization (MR) to elucidate causal relationships between the identified microbiota and major depression.
Results
Microbiome association analysis reveals association of thirteen taxa with depressive symptoms
The cohort characteristics are provided in Table 1. After exclusion of individuals using antidepressants and non-European subjects, 1,054 samples from RS and 1,539 samples from the HELIUS-study were included in the analyses (Table 1). The resulting microbiome data consisted of 17 phyla (for both cohorts), 33 classes for RS and 36 classes for HELIUS, 59 orders in RS and 61 orders for HELIUS, 116 families for RS and 108 families for HELIUS, and 439 genera for RS and 418 genera for HELIUS. In both cohorts, microbiome was dominated by phyla Firmicutes (77% in RS and 70% in HELIUS), Bacteroidetes (13% in RS and 21% in HELIUS), Actinobacteria (0.42% in RS and 0.42% in HELIUS) and Proteobacteria (0.48% in RS and 0.22% in HELIUS).
Alpha diversity was negatively associated with depressive symptoms in both RS (Shannon index; beta = −1.57, p value = 1.5 × 10−03) and HELIUS cohorts (Shannon index; beta = −0.64, p value = 2.84 × 10−02). Beta diversity showed significant association with depressive symptoms in RS (Permanova; R2 = 0.003, p value = 0.001) but not in the HELIUS cohort (R2 = 0.0005, p value = 0.51).
At taxonomic level, 24 genera, three microbial families, one class, two orders and a phylum were significantly (false discovery rate (FDR) < 5%) associated with depressive symptoms in the Rotterdam Study (Table 2, Source Data). We replicated these results in the HELIUS cohort for 12 genera, which were associated with depressive symptoms scores in the same direction (Table 2, Fig. 1). These include Sellimonas, Eggerthella, Ruminococcaceae (UCG002, UCG003, UCG005), Coprococcus3, Lachnoclostridium, Hungatella, LachnospiraceaeUCG001, Ruminococcusgauvreauiigroup, Eubacterium ventriosum and Subdoligranulum. Of the three microbial families significantly associated with depressive symptoms in RS, only family Ruminococcaceae was significantly associated with depressive symptoms in the HELIUS cohort. The direction of association was consistent for all associated taxa across both cohorts and the meta-analysis of results from both cohorts improved association p-values (Table 2). Of the 12 significantly associated genera 10 belong to the families Ruminococcaceae and Lachnospiraceae. All significantly associated genera belonging to the family Ruminococcaceae were depleted in those with higher depressive symptoms (Fig. 1). While most of the significantly associated genera within family Lachnospiraceae were increased in those reporting higher depressive symptoms (Fig. 1).
Table 2 Microbiota significantly associated with depressive symptoms
| Taxon | Rotterdam Study (N = 1054) | HELIUS (N = 1539) | Meta-analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | Se | p | fdr | Beta | Se | P-value | N | Zscore | P-value | |
| family.Christensenellaceae.id.1866 | −0.59 | 0.12 | 4.56E–07 | 8.04E–05 | −0.10 | 0.06 | 1.32E–01 | 2593 | −1.88 | 5.96E–02 |
| genus.ChristensenellaceaeR7group.id.11283 | −0.55 | 0.11 | 3.31E–07 | 8.04E–05 | −0.10 | 0.06 | 1.30E–01 | 2593 | −4.42 | 9.80E–06 |
| genus.RuminococcaceaeUCG005.id.11363 | −0.59 | 0.13 | 1.36E–05 | 1.57E–03 | −0.12 | 0.07 | 7.62E–02 | 2593 | −4.14 | 3.48E–05 |
| genus.RuminococcaceaeUCG010.id.11367 | −0.50 | 0.12 | 1.77E–05 | 1.57E–03 | −0.07 | 0.06 | 2.08E–01 | 2593 | −3.71 | 2.11E–04 |
| family.Ruminococcaceae.id.2050 | −1.59 | 0.38 | 2.51E–05 | 1.77E–03 | −0.52 | 0.24 | 3.11E–02 | 2593 | −4.35 | 1.38E–05 |
| genus.Coprococcus2.id.11302 | −0.29 | 0.08 | 2.74E–04 | 1.03E–02 | −0.02 | 0.04 | 5.79E–01 | 2593 | −2.75 | 6.02E–03 |
| genus.FamilyXIIIAD3011group.id.11293 | −0.74 | 0.20 | 2.56E–04 | 1.03E–02 | −0.06 | 0.10 | 5.78E–01 | 2593 | −2.76 | 5.78E–03 |
| genus.Lachnoclostridium.id.11308 | 0.57 | 0.16 | 3.11E–04 | 1.03E–02 | 0.27 | 0.11 | 1.80E–02 | 2593 | 4.12 | 3.76E–05 |
| genus.RuminococcaceaeUCG002.id.11360 | −0.42 | 0.12 | 3.21E–04 | 1.03E–02 | −0.16 | 0.07 | 2.33E–02 | 2593 | −4.04 | 5.30E–05 |
| genus.RuminococcaceaeUCG003.id.11361 | −0.51 | 0.15 | 4.60E–04 | 1.33E–02 | −0.25 | 0.08 | 3.10E–03 | 2593 | −4.51 | 6.42E–06 |
| class.Mollicutes.id.3920 | −0.39 | 0.12 | 8.63E–04 | 1.38E–02 | −0.01 | 0.05 | 8.04E–01 | 2593 | −2.32 | 2.06E–02 |
| genus.Eggerthella.id.819 | 0.65 | 0.19 | 6.28E–04 | 1.38E–02 | 0.31 | 0.12 | 1.18E–02 | 2593 | 4.12 | 3.80E–05 |
| genus.Hungatella.id.11306 | 0.77 | 0.22 | 6.73E–04 | 1.38E–02 | 0.34 | 0.14 | 1.34E–02 | 2593 | 4.07 | 4.65E–05 |
| genus.Ruminiclostridium6.id.11356 | −0.36 | 0.11 | 6.23E–04 | 1.38E–02 | −0.01 | 0.05 | 9.06E–01 | 2593 | −2.27 | 2.31E–02 |
| genus.RuminococcaceaeUCG014.id.11371 | −0.29 | 0.08 | 5.70E–04 | 1.38E–02 | −0.05 | 0.05 | 3.16E–01 | 2593 | −2.97 | 2.99E–03 |
| order.MollicutesRF9.id.11579 | −0.40 | 0.12 | 8.28E–04 | 1.38E–02 | 0.00 | 0.05 | 9.55E–01 | 2593 | −2.18 | 2.96E–02 |
| phylum.Tenericutes.id.3919 | −0.39 | 0.12 | 8.63E–04 | 1.38E–02 | −0.01 | 0.05 | 8.04E–01 | 2593 | −2.32 | 2.06E–02 |
| genus.Coprococcus3.id.11303 | −0.43 | 0.13 | 9.09E–04 | 1.39E–02 | −0.29 | 0.09 | 1.20E–03 | 2593 | −4.61 | 4.04E–06 |
| genus.LachnospiraceaeUCG001.id.11321 | −0.52 | 0.16 | 1.01E–03 | 1.48E–02 | −0.16 | 0.07 | 2.34E–02 | 2593 | −3.84 | 1.21E–04 |
| family.Micrococcaceae.id.636 | 1.53 | 0.47 | 1.26E–03 | 1.71E–02 | −0.14 | 0.16 | 3.75E–01 | 2593 | 2.92 | 3.55E–03 |
| genus..Ruminococcusgauvreauiigroup.id.11342 | −0.34 | 0.11 | 1.54E–03 | 2.01E–02 | −0.13 | 0.07 | 4.90E–02 | 2593 | −3.54 | 4.06E–04 |
| genus..Eubacteriumventriosumgroup.id.11341 | −0.49 | 0.16 | 2.09E–03 | 2.47E–02 | −0.17 | 0.08 | 4.20E–02 | 2593 | −3.53 | 4.19E–04 |
| genus.Sellimonas.id.14369 | 0.57 | 0.18 | 2.10E–03 | 2.47E–02 | 0.54 | 0.13 | 3.55E–05 | 2593 | 5.15 | 2.65E–07 |
| genus.Rothia.id.646 | 1.47 | 0.48 | 2.21E–03 | 2.52E–02 | 0.32 | 0.23 | 1.66E–01 | 2593 | 3.02 | 2.55E–03 |
| order.Micrococcales.id.510 | 1.41 | 0.47 | 2.58E–03 | 2.84E–02 | −0.21 | 0.15 | 1.68E–01 | 2593 | 2.96 | 3.09E–03 |
| genus.LachnospiraceaeNK4A136group.id.11319 | −0.41 | 0.14 | 2.80E–03 | 2.99E–02 | −0.08 | 0.08 | 3.48E–01 | 2593 | −2.63 | 8.56E–03 |
| genus.Subdoligranulum.id.2070 | −0.44 | 0.15 | 3.36E–03 | 3.48E–02 | −0.28 | 0.09 | 1.66E–03 | 2593 | −4.29 | 1.77E–05 |
| genus.RuminococcaceaeNK4A214group.id.11358 | −0.34 | 0.12 | 4.66E–03 | 4.70E–02 | −0.04 | 0.06 | 5.73E–01 | 2593 | −2.24 | 2.52E–02 |
| genus.PrevotellaceaeUCG001.id.11186 | −0.49 | 0.17 | 4.80E–03 | 4.71E–02 | −0.02 | 0.08 | 8.38E–01 | 2593 | −1.96 | 5.06E–02 |
Red dots depict negatively associated genera with depressive symptoms and blue ones depict positively associated genera with depressive symptoms. The outer most layer depicts the phylum level followed by class, order, family and genus levels.
Random forest analysis with RS as the training cohort and HELIUS as the testing cohort revealed RuminococcaceaeUCG005 as the most important genus in predicting depressive symptoms (Source Data), showing the highest percentage increase in mean squared error (%incMSE) in out of bag analysis. Other important predictors of depressive symptoms include ChristensenellaceaeR7group, Lachnoclostridium, Eggerthella, Sellimonas, and Hungatella, which overlap with the findings of the linear regression analysis in this study (Source Data). Further, important predictors identified by random forest analysis include Roseburia, Streptococcus, Bacteroides, Anaerotruncus, Dorea, Blautia, Veillonella, Desulfovibrio, Anaerostipes and Bifidobacterium, which replicate associations reported earlier28.
Mendelian Randomization (MR) analysis identifies a causal link between major depression and Eggerthella
Results of MR analysis are provided in the Source Data. With major depression as the exposure, Eggerthella, showed significant MR results under the IVW method (effect = 0.237, p value = 0.027) (Source Data). Tests for heterogeneity and horizontal pleiotropy were negative for Eggerthella (Supplementary Data 1, Supplementary Data 2). Further the effect estimates for Eggerthella was also consistent with the findings of this study, i.e., increase in the abundance of Eggerthella in those with higher depressive symptoms. Interestingly, the Steiger test for directionality suggests that Eggerthella is more likely to be causally associated with MDD (Supplementary Data 3). With microbiome as exposure, significant MR was observed for genus Sellimonas under the IVW method (effect = −0.046, p-value = 5.5*10−04) but effect estimate was inconsistent with the findings of our study (Supplementary Data 3).
Among the 87 depression-associated SNPs7 significant association was observed for one SNP rs17641524 with the genus Acidaminococcus after correction for multiple testing (Supplementary Data 4). No significant association was observed for the MDD GRS (Supplementary Data 5).
Discussion
In this large study of 2593 individuals profiled for depressive symptoms and fecal microbiome, we identified 12 genera and 1 microbial family associated with depressive symptoms. These include genera Sellimonas, Eggerthella, Ruminococcaceae (UCG002, UCG003, UCG005), Lachnoclostridium, Hungatella, Coprococcus, LachnospiraceaeUCG001, Ruminococcusgauvreauiigroup, Eubacterium ventriosum, Subdoligranulum and family Ruminococcaceae. Sellimonas, Eggerthella, Lachnoclostridium and Hungatella were more abundant in individuals with higher depressive symptoms. All other taxa were depleted in depression. Alpha diversity was significantly associated with depressive symptoms in both discovery and replication cohorts.
The intestinal bacterial strains Eggerthella, Subdoligranulum, Coprococcus and Ruminococcaceae have been reported to be associated with major depression in earlier studies. Eggerthella has been consistently found to be increased in depression and anxiety cases in 8 studies25,26,28, which is in line with the findings of our study. MR analysis suggests a causal link between MDD and Eggerthella, which requires further investigation. Also in line with our findings Subdoligranulum and Coprococcus were consistently found to be depleted in individuals with generalized anxiety disorder and depression in several studies28. In a recent study Coproccocus was depleted in rats that exhibited depressed behavior upon fecal transplantation from depressed human subjects29, suggesting that Coproccocus may have a causal impact on depression. Both Subdoligranulum and Coprococcus are involved in the production of butyrate26 and Subdoligranulum was found to be increased in omega 3 rich diet30. A previous meta-analysis shows that omega 3 fatty acids, more specifically eicosapentaenoic acid (EPA) supplementation are beneficial for depression31. Ruminococcaceae at genus and family levels have been found to be depleted in cases of both uni- and bipolar depression25,26,28,32,33,34. A similar pattern is observed in the study by Bosch et al. [NCOMMS-21-20669B] with several genera belonging to the family Ruminococcaceae depleted in those reporting higher depressive symptoms, which is again consistent with the results of our study.
Other findings of this study that have previously not been reported include association with genera Sellimonas, Lachnoclostridium, Hungatella, Eubacterium ventriosum, LachnospiraceaeUCG001, and Ruminococcusgauvreauiigroup. Sellimonas and Hungatella were positively associated with depressive symptoms. Sellimonas is the most significant finding of this study. It belongs to the family Lachnospiraceae and phylum Firmicutes. Species belonging to Sellimonas have been reported to be increased in inflammatory diseases including ankylosing spondylitis, atherosclerosis and liver cirrhosis35. Further, increased abundance of Sellimonas have been observed after dysbiosis36. Lachnoclostridium belongs to the family Lachnospiraceae. Higher levels of Lachnoclostridium were associated with increased depressive symptoms in our study and also consistent with the findings of the Bosch et al. study [NCOMMS-21-20669B]. Lachnoclostridium has previously found to be depleted in other psychiatric disorders including schizophrenia37 and autism38 and in patients with gastrointestinal tract neoplasms39. Hungatella belongs to the family Clostridiaceae and phylum Firmicutes. It has previously been associated with paleolithic diet and is known to produce the precursor molecule for trimethylamine-N-oxide (TMAO)40. TMAO has been implicated in cardio-vascular and neurological diseases including depression41,42. Eubacterium ventriosum belongs to the family Eubacteriaceae and has been found to be significantly depleted after traumatic brain injury in mice43. Major depression is a frequent complication of traumatic brain injury44. In our study we also observed depletion of Eubacterium ventriosum with the increase in depressive symptoms, which fits well with association with traumatic brain injury. In human studies Eubacterium ventriosum was found to be slightly more abundant in obese individuals45,46. Obesity is one of the most prevalent somatic comorbidities of major depressive disorder47,48 and is partly attributed to a side effect of selective serotonin reuptake inhibitors (SSRI). However, in our study we excluded those using antidepressants and adjusted for BMI in the linear regression analysis thus our finding is independent of the association with body weight. LachnospiraceaeUCG001, at species level, was found to be associated with anhedonia in mice49. Ruminococcusgauvreauii belongs to the family Ruminococcaceae and at species level was found to be increased in atherosclerotic conditions35. Interestingly depression is known to be causally associated with atherosclerosis50. It may be worth to investigate the genera Sellimonas and Ruminococcusgauvreauii as potential mediators in the relationship between depression and atherosclerotic conditions.
Most identified microbiota in our study show potential involvement in the synthesis of glutamate and butyrate (see Supplementary Data of Valles-Colomer et al. 2019)26. Eggerthella is further involved in the synthesis of serotonin and gamma aminobutyric acid (GABA). Glutamate is widely distributed in the brain and a major excitatory synaptic neurotransmitter51. It is known to be involved in regulating neuroplasticity, learning and memory52. Glutamate levels in plasma, serum, cerebrospinal fluid and brain tissue have been associated with mood and psychotic disorders and suicide53,54,55,56,57,58. With increasing evidence of its role in the etiology of depressive disorders, glutamate is rapidly becoming the novel therapeutic target for depressive disorders. Ketamine, for instance, has been shown to increase glutamate signaling in rodents and humans59,60 and has shown to reduce depressive symptoms rapidly61. Glutamate plays a role as a neurotransmitter in the enteric nervous system, which sustains the reciprocal influence between the gastrointestinal tract and the central nervous system8,62. Butyrate on the hand is a short chain fatty acid and modulates biological responses of host gastrointestinal health by acting as a histone deacetylase inhibitor and binding to specific G protein-coupled receptors (GPCRs)63. Butyrate can affect the gut-brain axis by enhancing the cholinergic neurons via epigenetic mechanisms64 and can cross the blood brain barrier and activate the vagus nerve and hypothalamus65,66. Sodium butyrate has shown anti-depressant effects in animal models of depression and mania67,68. Serotonin and GABA are both important neurotransmitters relevant to depression. Evidence suggests that serotonin may be the key neurotransmitter to the gut-brain axis17. Enteric nervous system accounts for >90% of the body’s serotonin production where it is produced by enterochromaffin cells and in the neurons of the enteric nervous system69. The neuronal production of serotonin is most critical for the development and motility of the enteric nervous system, affecting neurogenesis and guiding development of neurons expressing dopamine and GABA69,70,71. Although serotonin produced by the gut cannot cross the blood-brain barrier72, it can affect the blood-brain barrier permeability, which can lead to inflammation of the brain73. Further, vagus nerve stimulation by the gut microbiota can alter concentration of serotonin, GABA and glutamate within the brain in animals and humans42,74 and germ-free male mice exhibit anxiety-like behaviors and altered serotonin abundance in the brain14. GABA is the main inhibitory neurotransmitter of the central nervous system that counterbalances the action of glutamate75. Low levels of GABA are linked to depression and mood disorders75. Animal studies show that gut microbiota can alter GABA activity in the brain through the vagus nerve76. While each of the metabolites mentioned above are highly relevant for depression, most are known to be unable to cross the blood-brain barrier. However, an increasing number of animal studies show that the peripheral production of neurotransmitters by the gut microbiome can alter brain chemistry and therefore influence mood and behavior42.
In the current study, we aimed to identify gut microbiota associated with depressive symptoms in the general population. The strengths of our study include a large sample, controlling for most known confounders including comorbid conditions, performing analysis in individuals free of anti-depressive medication and finally the use of quantitative depression scales. A large study consisting of 252,503 individuals from 68 countries showed that subthreshold depressive disorders produce significant decrements in health and do not qualitatively differ from full-blown episodes of depression77. Use of rating scales is thus more powerful in omics association studies78. There may have been a loss of statistical power as the depression assessment scales were different in the discovery and replication cohorts. Further, despite the use of the largest GWAS for both microbiome and depression, the MR analysis lacked power. There are 87 SNPs identified for depression, however, their effect on depression is small (individual odds ratio <1.05, combined odds ratio <2.0), which makes unlikely that the individual genetic variants show association with microbiome. For microbiome, there were no SNPs significantly associated at the genome-wide level. Therefore, we had to lower the threshold to 10−05 to identify at least more than one independent instrument for the identified microbiota. This limits the value of the MR. Another limitation of this study is using different methods for stool sampling and sequencing variable regions. These factors might influence the microbial profiles substantially. For example, reads generated by the V4 primer pair showed a higher alpha diversity of the gut microbial community than V1-V2 and V3-V479. In addition, a recent study showed significant differences in bacterial composition that result from collecting stool samples using different stool collection methods compared to immediate freezing80. This may have a negative impact on statistical power. However, despite the differences there is a significant overlap and consistency in effect estimates between the discovery and the replication cohorts. The overlapping results of this study are, therefore, of greater importance, as they are consistent despite methodological differences. It is interesting to note that despite the fact that we replicate most of our findings in the European participants of the HELIUS cohort, there’s only partial overlap with the findings of the study by Bosch et al. [NCOMMS-21-20669B]. However, the lack of significant ethnic differences in that study suggests that non-replication between cohorts (as inferred by p-value testing) likely is in the realm of normal sample variation and coupled to small effect sizes, i.e., may (at least partially) reflect Type 2 statistical error. Another difference is the classification method used for taxonomic identification of bacteria. Bosch et al. uses Amplicon Sequence Variants in 3,211 participants from 6 ethnic groups [NCOMMS-21-20669B], while this study uses closed reference OTU clustering with the same SILVA database in only European participants. Nevertheless, irrespective of the above methodological differences reproduced associations are observed for Lachnoclostridium, Coproccocus and Ruminoccocaceae [NCOMMS-21-20669B] suggesting robust association of depressive symptoms with these taxa.
To summarize, we have identified several bacteria at genera level that might influence depression in humans. We confirm the association of Eggerthella, Coprococcus, Subdoligranulum and family Ruminococcaceae and identify bacteria including Sellimonas, Lachnoclostridium, Hungatella, Ruminococcus, Subdoligranulum, LachnospiraceaeUCG001, Eubacterium ventriosum and Ruminococcusgauvreauiigroup. These bacteria are involved in the synthesis of glutamate, butyrate, serotonin and GABA, which are the key neurotransmitters relevant for depression.
Methods
Study population
The discovery cohort includes 1054 participants from the Rotterdam Study who were not using anti-depressants at the time of assessment. The Rotterdam Study is a population-based cohort study from the well-defined Ommoord district within Rotterdam, The Netherlands. It is designed to investigate occurrence and determinants of diseases in the elderly81. Initially, the RS included 7,983 participants in 1990 who underwent an at-home interview, extensive physical examination at baseline and during follow-up examinations that occur every 3–4 years (RS-I). The RS was extended with two more cohorts in 2000 (RS-II) and 2005 (RS-III) and contains a total of 14,926 participants. In this study we used the data of individuals from the second follow up of the third Rotterdam Study cohort (RS-III-2) as these individuals were profiled for the gut microbiome. The Rotterdam Study (RS-III-2) consists of individuals of European background. The RS is approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The RS was entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians. Participants were not compensated for their participation.
The replication cohort included 1539 participants from the Healthy Life in an Urban Setting (HELIUS) cohort. The HELIUS cohort is a multiethnic cohort consisting of individuals of Dutch, Surinamese, Ghanaian, Turkish and Moroccan origin from Amsterdam. People in the age range of 18–70 years were randomly sampled, stratified by ethnic origin through the municipality register of Amsterdam. This register contains data on country of birth of citizens and of their parents, thus allowing for sampling based on the widely accepted Dutch standard indicator for ethnic origin82. The Dutch sample includes people who were born in the Netherlands and whose parents were born in the Netherlands. The current study used data from Dutch samples only. The Medical Ethics Committee of the Amsterdam UMC, location AMC approved the study protocols. Written informed consent was obtained from all participants, who were not compensated for their participation.
Fecal sample collection and microbiome profiling
Detailed description on how the gut microbiome composition was generated at RS-III-2 (2012-2013) and in the HELIUS cohort are described elsewhere83,84. Briefly, in RS, participants were instructed to collect a stool sample at their home in sterile tubes and to send the sample by regular mail to the research location of Erasmus Medical Center (EMC), Rotterdam, the Netherlands. Upon arrival at Erasmus MC, samples were checked and stored at −20 °C. Samples, which were underway for more than 3 days, were excluded84. Subsequently, an automated stool DNA isolation kit (Diasorin, Saluggia, Italy) was used to isolate bacterial DNA from approximately 300 mg stool aliquot using a bead-beating step. The V3 and V4 hypervariable regions of the bacterial 16 S rRNA gene were amplified and sequenced on an Illumina MiSeq platform with the V3 kit (2 × 300 bp paired-end reads; Illumina).
Participants from the HELIUS-study were given a stool collection tube and requested to collect a stool sample and bring their samples to the research location within 6 h after collection and if not possible kept in their freezer overnight and bring it to the research location the next morning. At the research location, the samples were temporarily stored at −20 °C until daily transportation to the Amsterdam Medical Center (AMC), Amsterdam, the Netherlands, where the samples were checked and stored at −80 °C. Total genomic DNA was extracted from a 150 mg aliquot using a repeated bead beating method85. Briefly, fecal samples were bead beated twice and after each bead-beating cycle, samples were heated at 95 °C for 5 min. Supernatants from two extractions were pooled and DNA was purified using the QIAamp DNA Mini kit (QIAGEN Benelux B.V., Venlo, The Netherlands) on the QIAcube (QIAGEN) instrument using the procedure for human DNA analysis.
The composition of fecal microbiota was determined by sequencing the V4 region of the 16 S rRNA gene on a MiSeq system (Illumina) with 515 F and 806 R primers designed for dual indexing (42) and the V2 kit (2 × 250 bp paired-end reads; Illumina). Raw sequencing data from both cohorts were run through the same microbiome-profiling pipeline to harmonize the microbiome data.
Reads were subsampled at 10,000 reads per sample. Taxonomy was assigned using the standard profiling pipeline developed by the MiBioGen consortium86. Briefly, we implemented the 16 S data processing pipeline, which comprised closed reference OTU clustering without a de-noising step based on the naive Bayesian classifier from the Ribosomal Database Project (version 2.12) and the most recent (version 128), full, SILVA database. We only analyzed taxonomical results at genus and higher taxonomic levels. Alpha diversity indices such as species richness, Shannon index and Inverse Simpson were calculated at the genus-level. We calculated Bray-Curtis distances based on absolute abundance of microbial communities at genus level to measure beta-diversity. For single taxon analyses, taxa that were present in less than 3% of the sample size (each cohort separately) and taxa with read counts less than 0.005% of the total number of reads were excluded. Taxa abundances (absolute counts) were then log transformed (to the absolute values 1 was added before log-transformation).
Depression assessment
In RS depressive symptoms were assessed using the 20-item version of the Center for Epidemiological Studies-Depression (CES-D) scale87. CES-D is a self-report measure of symptoms experienced during the prior week. It has been shown to be relatively stable over time and covers the major dimensions of depression including depressed mood, feelings of guilt and worthlessness, feelings of helplessness and hopelessness, psychomotor retardation, loss of appetite and sleep disturbance88. The total score ranges from 0 to 60, with higher scores indicating a greater burden of depressive symptoms. The CES-D detects current MDD cases with high sensitivity and specificity. We used the depression assessment from RS-III-2 (the same time as the collection of the feces).
For participants of the HELIUS cohort, depression was assessed using the Patient Health Questionnaire (PHQ-9) design89. PHQ-9 scores each of the DSM-IV criteria as “0” (not at all) to “3” (nearly every day). The total score ranges from 0 to 21, with higher scores indicating severity of depression. A PHQ-9 score of ≥10 has a sensitivity and specificity of 88% to detect major depression. Individuals with ethnic background other than Europeans and individuals using antidepressants were excluded.
Statistics and reproducibility
Overall, no statistical method was used to predetermine sample size. No data were excluded from the analysis and the experiments were not randomized. The investigations were not blinded to allocation during experiments and outcome assessment.
Microbiome association analysis
To test the association of depressive symptom scores with alpha diversity and individual taxa we used linear regression models using depression scores as the outcome and alpha diversity and taxa (log+1 transformed) as independent variables adjusting for several covariates including sex, age, alcohol use, body mass index (BMI), smoking, medication use (proton pump inhibitors (PPI), metformin, lipid-lowering and antibiotics) and technical covariates including time in mail and batch (in case of RS cohort). Association of the depression scores with microbiome beta-diversity was performed using permutation analysis of variance (PERMANOVA) in R-package “vegan” using the same model as described above.
Results from the discovery and replication cohorts were combined in a meta-analysis using METAL software90. Since the depressive symptoms assessment scales were different in the discovery and replication cohorts, we used sample-size weighted meta-analysis to combine the results. Adjustment for multiple testing was performed using false discovery rate (FDR) using Benjamini-Hochberg correction.
Further, we performed a random forest regression analysis using Breiman’s random forest algorithm91 for regression implemented in the “randomForest” library of the R software. Random forest is a tree-based machine learning algorithm that captures non-linear relationships and can deal with highly correlated input data by leveraging the power of multiple decision trees in order to control overfitting problem. In particular, each tree in the ensemble is built from a bootstrapped sample from the training sample and each node of the tree works on a random subset of the total feature. For this analysis RS stool microbiome profiles were used as predictors and depression scores as response for the training data set, while the HELIUS stool microbiome profiles and depression scores as predictors and response as the test data. Hyperparameters of the model including number of trees (ntree = 500) and number of variables randomly sampled as candidates at each split (mtry = 100) were tuned to give the best performance based on the increase in mean square error (%IncMSE) calculated from out-of-bag samples. In addition, we set the number of times the out of bag data is permuted per tree for assessing variable importance to 100 (nPerm = 100).
Mendelian randomization (MR) analysis
To ascertain causal links between the identified microbiota and major depressive disorder (MDD) we performed two-sample MR analysis using the results of the largest genome-wide association studies of both microbiome and major depression7,92. For major depression we used genome-wide significant single nucleotide polymorphisms (SNPs) as instruments7 (Source Data). For microbiome there were none to a very few SNPs that were genome-wide significantly associated with the identified microbiota, so we used SNPs with a p-value <10−05 as instruments (Source Data). MR analysis was performed using the “TwoSampleMR” library93 of the R software. Linkage disequilibrium pruning of the SNPs was performed using the ‘clump_data’ option with the clump r2 of 0.01 to identify independent instruments. MR report was generated using the ‘mr_report’ option. This method reports results from the weighted median, simple and weighted mode, Inverse variance weighted (IVW) and Egger methods. Variance explained (R2) per instrument for both the exposures and the outcomes were generated using the ‘add_rsq’ option.
We further examined the microbiome-wide association of each of the 87 SNPs associated with depression using the microbiome GWAS summary statistics from Kurilshikov et al.92 to identify the gut microbiota associated with these SNPs. Finally, we tested the association of the genetic risk score combining the summary level data of the 87 SNPs for each microbiota in an unweighted genetic risk score using inverse-weighted method in the ‘rmeta’ package of R software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All relevant data supporting the key findings of this study are available within the article and its supplementary files. Full results of the genus-level linear models (Fig. 1, and Table 2), Random forest analyses and Mendelian randomization are supplied as Source Data. Individual-level data of Rotterdam Study and HELIUS Study are not publicly available due to privacy regulations (GDPR). Also, raw 16 S sequencing data from the Rotterdam Study is not publicly available as sharing of participant data, either pseudo-anonymized or anonymized, was not part of the informed consent. Raw 16 S sequencing data from HELIUS participants is available through European Genome-Phenome archive (EGAD00001004106). Rotterdam Study data are available upon request to the data manager Frank van Rooij (f.vanrooij@erasmusmc.nl) and subject to local rules and regulations. This includes submitting a proposal to the management team of RS, where upon approval, analysis needs to be done on a local server with protected access, complying with GDPR regulations. Source data are provided with this paper.
Code availability
The codes used for the analyses in this study are available at https://github.com/Djawad-Radj/Microbiome_Depression.
References
-
Bromet, E. et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 9, 90 (2011).
-
Salari, N. et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob. Health 16, 57 (2020).
-
Papakostas, G. I. & Fava, M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur. Neuropsychopharmacol. 19, 34–40 (2009).
-
Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366 (2018).
-
Morley, J. E. The effectiveness and harms of antidepressants. J. Am. Med. Dir. Assoc. 18, 279–281 (2017).
-
Sullivan, P. F., Neale, M. C. & Kendler, K. S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry 157, 1552–1562 (2000).
-
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343 (2019).
-
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
-
Dinan, T. G. & Cryan, J. F. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol. Motil. 25, 713–719 (2013).
-
Cryan, J. F. & Dinan, T. G. Microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat. Rev. Gastroenterol. Amp; Hepatol. 12, 494 (2015).
-
Desbonnet, L. et al. Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148 (2014).
-
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 (2013).
-
Bailey, M. T. et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407 (2011).
-
Clarke, G. et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 (2013).
-
El Aidy, S. et al. The microbiota and the gut-brain axis: insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Beneficial Microbes 3, 251–259 (2012).
-
Möhle, L. et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 15, 1945–1956 (2016).
-
O’Mahony, S. M. et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Res. 277, 32–48 (2015).
-
Ogbonnaya, E. S. et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry 78, e7–e9 (2015).
-
Park, A. J. et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil.: Off. J. Eur. Gastrointest. Motil. Soc. 25, 733–e575 (2013).
-
Sudo, N. et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275 (2004).
-
Yano, J. M. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276 (2015).
-
Messaoudi, M. et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764 (2011).
-
Kelly, J. R. et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118 (2016).
-
Steenbergen, L. et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behav., Immun. 48, 258–264 (2015).
-
Cheung, S. G. et al. Systematic review of gut microbiota and major depression. Front. Psychiatry 10, 34 (2019).
-
Valles-Colomer, M. et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632 (2019).
-
Fung, T. C. et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 4, 2064–2073 (2019).
-
Simpson, C. A. et al. The gut microbiota in anxiety and depression – A systematic review. Clin. Psychol. Rev. 83, 101943 (2021).
-
Knudsen, J. K. et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci. Rep. 11, 21869 (2021).
-
Noriega, B. S. et al. Understanding the impact of Omega-3 rich diet on the gut microbiota. Case Rep. Med 2016, 3089303 (2016).
-
Liao, Y. et al. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 9, 190 (2019).
-
Hu, S. et al. Gut microbiota changes in patients with bipolar depression. Adv. Sci. 6, 1900752 (2019).
-
Li, S. et al. The role of bacteria and its derived metabolites in chronic pain and depression: Recent findings and research progress. Int. J. Neuropsychopharmacol 23, 26–41 (2020).
-
Liu, R. T. et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 88, 308–324 (2020).
-
Nayfach, S. et al. New insights from uncultivated genomes of the global human gut microbiome. Nature 568, 505–510 (2019).
-
Muñoz, M. et al. Comprehensive genome analyses of <em>Sellimonas intestinalis</em>, a potential biomarker of homeostasis gut recovery. bioRxiv, 2020: p. 2020.04.14.041921.
-
Zheng, P. et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 5, eaau8317 (2019).
-
Ma, B. J. et al. Altered gut microbiota in chinese children with autism spectrum disorders. Front. Cellular Infect. Microbiol. 9, 40 (2019).
-
Youssef, O. et al. Stool microbiota composition differs in patients with stomach, colon, and rectal neoplasms. Digestive Dis. Sci. 63, 2950–2958 (2018).
-
Genoni, A. et al. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur. J. Nutr. 59, 1845–1858 (2020).
-
Janeiro, M. H. et al. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10, 1398 (2018).
-
Caspani, G. et al. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Micro. Cell 6, 454–481 (2019).
-
Treangen, T. J. et al. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 9, 2757 (2018).
-
Jorge, R. E. et al. Major depression following traumatic brain injury. Arch. Gen. Psychiatry 61, 42–50 (2004).
-
Kasai, C. et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 15, 100 (2015).
-
Tims, S. et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 7, 707–717 (2013).
-
de Wit, L. et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res 178, 230–235 (2010).
-
Luppino, F. S. et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 67, 220–229 (2010).
-
Yang, C. et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 9, 57 (2019).
-
Jee, Y. H. et al. Cohort study on the effects of depression on atherosclerotic cardiovascular disease risk in Korea. BMJ Open 9, e026913 (2019).
-
Mathews, D. C., Henter, I. D. & Zarate, C. A. Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 72, 1313–1333 (2012).
-
Malenka, R. C. & Nicoll, R. A. Long-term potentiation–a decade of progress? Science 285, 1870–1874 (1999).
-
Frye, M. A. et al. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol. Psychiatry 61, 162–166 (2007).
-
Holemans, S. et al. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res 616, 138–143 (1993).
-
Kim, J. S. et al. Increased serum glutamate in depressed patients. Arch. Psychiatr. Nervenkr (1970) 232, 299–304 (1982).
-
Levine, J. et al. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 47, 586–593 (2000).
-
Mitani, H. et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1155–1158 (2006).
-
Nowak, G., Ordway, G. A. & Paul, I. A. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res 675, 157–164 (1995).
-
Duman, R. S., Sanacora, G. & Krystal, J. H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90 (2019).
-
Chowdhury, G. M. et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol. Psychiatry 22, 120–126 (2017).
-
McGirr, A. et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J. Psychiatr. Res 62, 23–30 (2015).
-
Mazzoli, R. & Pessione, E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microbiol 7, 1934 (2016).
-
de Clercq, N. C. et al. Gut microbiota in obesity and undernutrition. Adv. Nutr. 7, 1080–1089 (2016).
-
Soret, R. et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138, 1772–1782 (2010).
-
Gagliano, H. et al. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology 79, 75–82 (2014).
-
Liu, H. et al. Butyrate: a double-edged sword for health? Adv. Nutr. 9, 21–29 (2018).
-
Valvassori, S. S. et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr. Neurovasc Res. 12, 312–320 (2015).
-
Resende, W. R. et al. Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav. Pharm. 24, 569–79. (2013).
-
Gershon, M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21 (2013).
-
Li, Z. et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009 (2011).
-
Li, Z. S. et al. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 24, 1330–1339 (2004).
-
Berger, M., Gray, J. A. & Roth, B. L. The expanded biology of serotonin. Annu Rev. Med. 60, 355–366 (2009).
-
Lawther, B. K., Kumar, S. & Krovvidi, H. Blood-brain barrier. Continuing Educ. Anaesth. Crit. Care Pain. 11, 128–132 (2011).
-
Ressler, K. J. & Mayberg, H. S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 10, 1116–1124 (2007).
-
Lydiard, R. B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 64, 21–27 (2003).
-
Bravo, J. A. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 108, 16050–16055 (2011).
-
Ayuso-Mateos, J. L. et al. From depressive symptoms to depressive disorders: the relevance of thresholds. Br. J. Psychiatry 196, 365–371 (2010).
-
Story Jovanova, O. et al. DNA methylation signatures of depressive symptoms in middle-aged and elderly persons: meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry 75, 949–959 (2018).
-
Chen, Z. et al. Impact of preservation method and 16S rRNA hypervariable region on gut microbiota profiling. mSystems. 4. https://doi.org/10.1128/mSystems.00271-18 (2019).
-
Jones, J. et al. Fecal sample collection methods and time of day impact microbiome composition and short chain fatty acid concentrations. Sci. Rep. 11, 13964 (2021).
-
Ikram, M. A. et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur. J. Epidemiol. 32, 807–850 (2017).
-
Snijder, M. B. et al. Cohort profile: the Healthy Life in an Urban Setting (HELIUS) study in Amsterdam, The Netherlands. BMJ Open 7, e017873 (2017).
-
Deschasaux, M. et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 24, 1526–1531 (2018).
-
Radjabzadeh, D. et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 10, 1040 (2020).
-
Mobini, R. et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 19, 579–589 (2017).
-
Wang, J. et al. Meta-analysis of human genome-microbiome association studies: the MiBioGen consortium initiative. Microbiome 6, 101 (2018).
-
Lewinsohn, P. M., Seeley, J. R., Roberts, R. E. & Allen, N. B. Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging 12, 277–287 (1997).
-
Hek, K. et al. A genome-wide association study of depressive symptoms. Biol. Psychiatry 73, 667–678 (2013).
-
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
-
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinforma. (Oxf., Engl.) 26, 2190–2191 (2010).
-
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
-
Kurilshikov, A. et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet 53, 156–165 (2021)
-
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 7, e34408 (2018).
Acknowledgements
The Rotterdam Study is a population-based cohort study from the well-defined Ommoord district within Rotterdam, The Netherlands. It is designed to investigate occurrence and determinants of diseases in the elderly. The RS cohort is approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272-159521-PG). The Rotterdam Study was entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. We thank all participants and all others, who made this study possible.
The HELIUS study is conducted by the Amsterdam University Medical Centers, location AMC and the Public Health Service of Amsterdam. Both organisations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation, the Netherlands Organization for Health Research and Development (ZonMw), the European Union (FP-7), and the European Fund for the Integration of non-EU immigrants (EIF). We are most grateful to the participants of the HELIUS study and the management team, research nurses, interviewers, research assistants and other staff who have taken part in gathering the data of this study. Grant numbers:
– ZonMW Memorabel: 733050814 (C.M.v.D.)
– Dutch Heart Foundation: 2010T084 (K.S)
– ZonMw: 200500003 (K.S)
– European Union (FP-7): 278901 (K.S)
– European Fund for the Integration of non-EU immigrants (EIF): 2013EIF013 (K.S)
– European Union (H2020) Research Innovation Action (RIA). Grant agreement ID: 848146 (JA Bosch)
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Mary Kimmel, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
source by Djawad Radjabzadeh, Jos A. Bosch, André G. Uitterlinden, Aeilko H. Zwinderman, M. Arfan Ikram, Joyce B. J. van Meurs, Annemarie I. Luik, Max Nieuwdorp, Anja Lok, Cornelia M. van Duijn, Robert Kraaij & Najaf Amin